Several molecular events are associated with the transition to castration resistance – most commonly alterations in the androgen receptor (AR), but also in DNA repair pathways such as homologous recombination repair (HRR).1,2 In patients with mCRPC, the prevalence of somatic and germline HRR mutations is approximately 20-25% and 12%, respectively,3 and as such guidelines recommend that all patients with metastatic disease undergo testing for HRR alterations.4 In HRR-mutated (HRRm) patients, the loss of function of this pathway can be exploited through the use of poly(ADP-ribose) polymerase inhibitors (PARPis) resulting in cell death due to prevention of DNA repair – a concept known as synthetic lethality. As such, PARPis are approved for use in BRCA-mutated mCRPC that has been previously treated with an androgen receptor pathway inhibitor (ARPI).
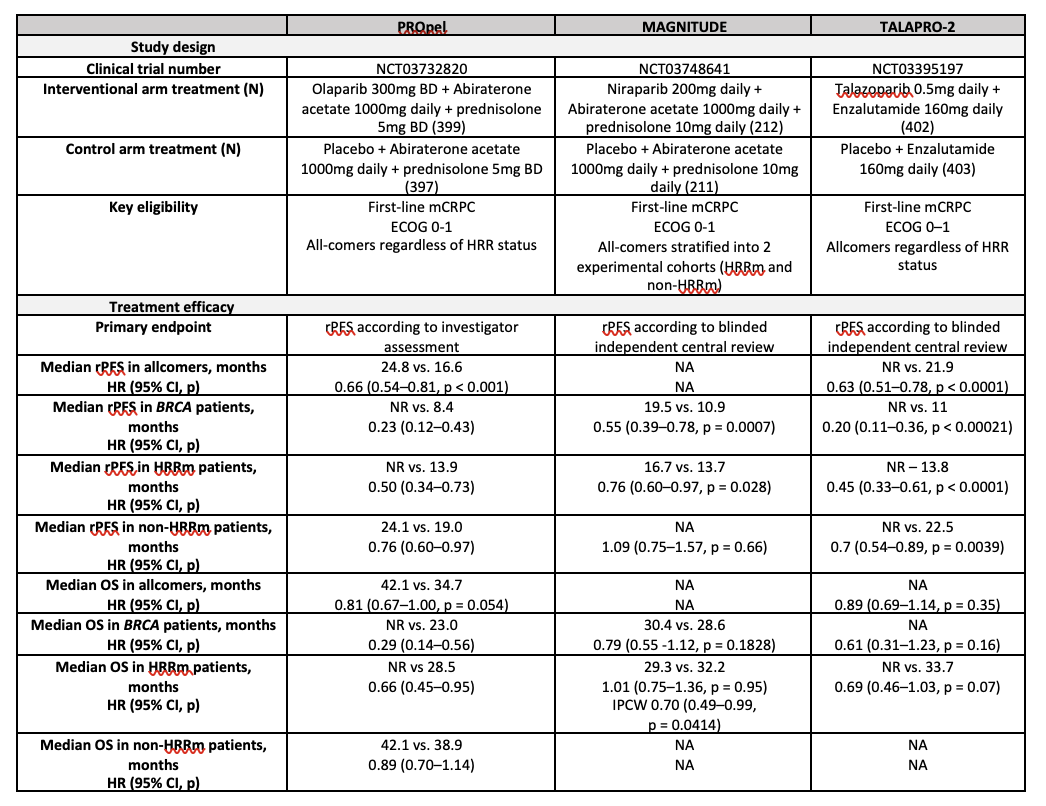
Concurrent inhibition of AR and PARP with an ARPI-PARPi combination in patients with mCRPC leads to therapeutic lethal synergy due to several mechanisms.5 ARPIs suppress AR activity, upregulate PARP enzymes, and downregulate HRR gene expression, inducing a phenotype resembling an HRR-mutant state. PARPis suppress AR transcriptional activity and potentially may attenuate some mechanisms of resistance to ARPIs. Three pivotal phase III randomised trials have therefore evaluated various ARPI-PARPi combinations in patients with mCRPC – PROpel (olaparib and abiraterone acetate/prednisolone), MAGNITUDE (niraparib and abiraterone acetate/prednisolone), and TALAPRO-2 (talazoparib and enzalutamide) (Table 1).6-11
Though each trial differed in terms of the trial design, drugs used, patient populations, and method of HRR mutation testing, they all demonstrated a differential effect on radiographic progression-free survival (rPFS) based on the presence and type of HRR mutation. In all three studies, a clear hierarchy of benefit was observed, with the BRCA-mutant population deriving the greatest benefit from an ARPI-PARPi combination when compared to the control arm (ARPI-placebo), followed by the overall HRRm cohort. HRR-unselected or non-HRRm patients derived the lowest magnitude of benefit. Further, in the PROpel and MAGNITUDE trial subgroup analyses, patients who were taxane-naïve had longer rPFS than those who had received prior docetaxel. Given these differential responses, and the potential impact of prior treatments such as docetaxel on efficacy, early screening for HRR mutations is crucial to guide treatment choices. As expected, toxicity was increased with combination therapy, with high-grade anaemia, in particular, a frequent adverse event across the three trials. This was particularly the case in the TALAPRO-2 trial, with talazoparib having the most potent PARP1-trapping capacity.
Table 1: Pivotal Phase 3 Clinical Trials Evaluating PARPi and ARPI in mCRPC
The treatment landscape has recently evolved to include an ARPI as standard first-line treatment for metastatic hormone-sensitive prostate cancer (mHSPC), obfuscating the interpretation of the PROpel, MAGNITUDE, and TALAPRO-2 that were largely conducted in ARPI-naïve patients. Several trials currently underway in both HRR-selected and -unselected populations in mHSPC, combined with the development of more selective (and potentially less haemotoxic) PARPi’s, will hopefully provide further evidence of the benefits of ARPI-PARPi combinations in prostate cancer. Identification of patients and subgroups who derive the greatest benefit from treatment intensification will optimise the treatment paradigm moving forwards.
Written by:
- Louise Kostos, PhD candidate, Consultant Medical Oncologist, Department of Medical Oncology, Peter MacCallum Cancer Centre, Sir Peter MacCallum Cancer Centre, University of Melbourne, Melbourne, Australia
- Arun A. Azad, MBBS, PhD, FRACP, Associate Professor, Department of Medical Oncology, Peter MacCallum Cancer Centre, Sir Peter MacCallum Cancer Centre, University of Melbourne, Melbourne, Australia
- Watson PA, Arora VK, Sawyers CL. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nature Reviews Cancer. 2015;15(12):701-11.
- Cai M, Song X-L, Li X-A, Chen M, Guo J, Yang D-H, et al. Current therapy and drug resistance in metastatic castration-resistant prostate cancer. Drug Resistance Updates. 2023;68:100962.
- Pritchard CC, Mateo J, Walsh MF, De Sarkar N, Abida W, Beltran H, et al. Inherited DNA-Repair Gene Mutations in Men with Metastatic Prostate Cancer. N Engl J Med. 2016;375(5):443-53.
- Network NCC. NCCN Guidelines Version 4.2024
- Kostos L, Tran B, Azad AA. Combination of PARP Inhibitors and Androgen Receptor Pathway Inhibitors in Metastatic Castration-Resistant Prostate Cancer. Drugs. 2024.
- Clarke NW, Armstrong AJ, Thiery-Vuillemin A, Oya M, Shore N, Loredo E, et al. Abiraterone and Olaparib for Metastatic Castration-Resistant Prostate Cancer. NEJM Evidence. 2022;1(9):EVIDoa2200043.
- Agarwal N, Azad AA, Carles J, Fay AP, Matsubara N, Heinrich D, et al. Talazoparib plus enzalutamide in men with first-line metastatic castration-resistant prostate cancer (TALAPRO-2): a randomised, placebo-controlled, phase 3 trial. The Lancet. 2023;402(10398):291-303.
- Chi K, Rathkopf D, Smith M, Efstathiou E, Attard G, Olmos D, et al. Phase 3 MAGNITUDE study: First results of niraparib (NIRA) with abiraterone acetate and prednisone (AAP) as first-line therapy in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) with and without homologous recombination repair (HRR) gene alterations. Journal of Clinical Oncology. 2022;40:12-.
- Chi KN, Rathkopf D, Smith MR, Efstathiou E, Attard G, Olmos D, et al. Niraparib and Abiraterone Acetate for Metastatic Castration-Resistant Prostate Cancer. J Clin Oncol. 2023;41(18):3339-51.
- Saad F, Clarke NW, Oya M, Shore N, Procopio G, Guedes JD, et al. Olaparib plus abiraterone versus placebo plus abiraterone in metastatic castration-resistant prostate cancer (PROpel): final prespecified overall survival results of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2023;24(10):1094-108.
- Fizazi K, Azad AA, Matsubara N, Carles J, Fay AP, De Giorgi U, et al. First-line talazoparib with enzalutamide in HRR-deficient metastatic castration-resistant prostate cancer: the phase 3 TALAPRO-2 trial. Nature Medicine. 2024;30(1):257-64.


