Tissue studies have confirmed 5-ARIs induce >90% decreases in intraprostatic DHT levels.7,8 This decline is nearly identical to that observed after androgen deprivation therapy (ADT) with a luteinizing hormone-releasing hormone agonist or antagonist.9
Finasteride was studied in the Prostate Cancer Prevention Trial (PCPT). In this randomized controlled trial (RCT) of 18,000 men, those in the finasteride arm had a 25% reduction in PCa incidence.3 Dutasteride was studied in the Reduction by Dutasteride of PCa Events (REDUCE) trial. In this RCT of 6,729 men, there was a 23% reduction in PCa incidence in the dutasteride arm.1 These risk reductions translate into a number needed to treat (NNT) to prevent one case of PCa of about 20.1–3
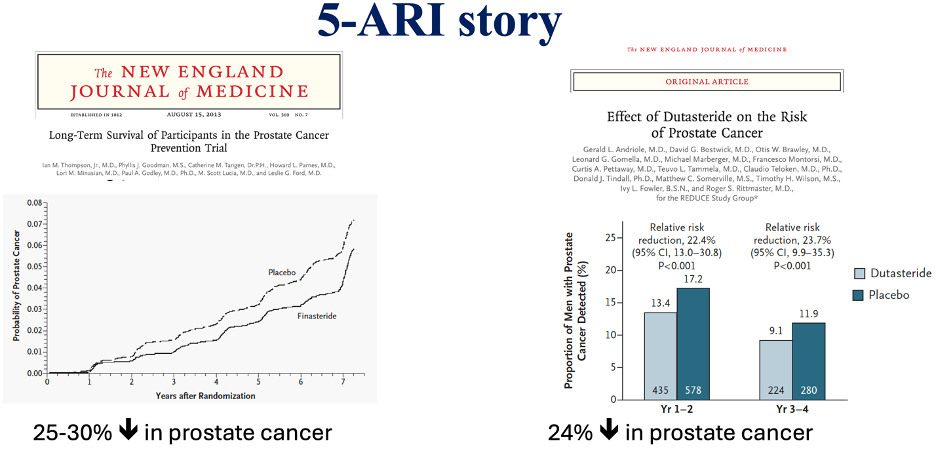
These two trials have been criticized because of the increased risk of high-grade tumors observed among 5-ARI users. In the PCPT, the proportion of high-grade tumors (Gleason ≥7) was 27% higher in the finasteride arm,3 and in the REDUCE trial, though no significant difference in Gleason ≥7 tumors was reported, there was a trend toward increased risk in Gleason ≥8 tumors (29 (0.9%) vs. 19 (0.6%); P = 0.15).1,10
This could be attributed to several theories, including the volume-grade bias, which suggests that a larger prostate volume results in fewer high-grade cancers being diagnosed at biopsy. However, the fact that prostatectomy results are similar for these men suggests a sampling artifact, as the distribution of cancer grade does not correlate with prostate volume.11 Furthermore, 5-ARIs might increase the sensitivity of PSA and digital rectal examination (DRE) for detecting high-grade disease, or they could potentially induce the development of high-grade tumors.
As a result, the FDA Oncology Drugs Advisory Committee (ODAC) reviewed the data and issued a black box warning against the use of 5-ARIs for cancer prevention. The concern about placing otherwise healthy men on 5-ARIs, given the potential risk of inducing high-grade disease and increasing prostate cancer mortality, has deterred physicians from widely adopting these medications.11
The aim of our study was to examine the association between 5-ARI use prior to PCa diagnosis and survival outcomes in a population-based cohort with long-term follow-up and detailed pathological information.
We used the following databases to capture newly diagnosed PCa in Ontario, Canada, capture treatment, demographics and vital status and outpatient prescriptions of 5-ARI:
- Ontario Cancer Registry (OCR) – captures prostate cancers
- OHIP - tracks claims paid to physicians (e.g. surgery or radiotherapy)
- Registered Persons Data Base (RPDB) - demographics and vital status
- Ontario Drug Benefit (ODB) database - outpatient prescriptions for patients ≥65
Between January 2003 and October 2017, we reviewed 128 017 patients with PCa and identified a total of 65 626 patients aged 65 years or older who received a diagnosis of PCa and who were treated with radiation therapy, active surveillance, ADT, or radical prostatectomy. Of whom 46 337 had pathology reports available. After exclusions, including 26 399 missing a complete set of variables of interest, 19,938 formed our final cohort.

For our statistical analysis, we used:
- Stabilized inverse probability treatment weights (IPTWs) based on the probability of receiving 5-ARIs and calculated from a logistic regression model adjusting for prognostic covariates:
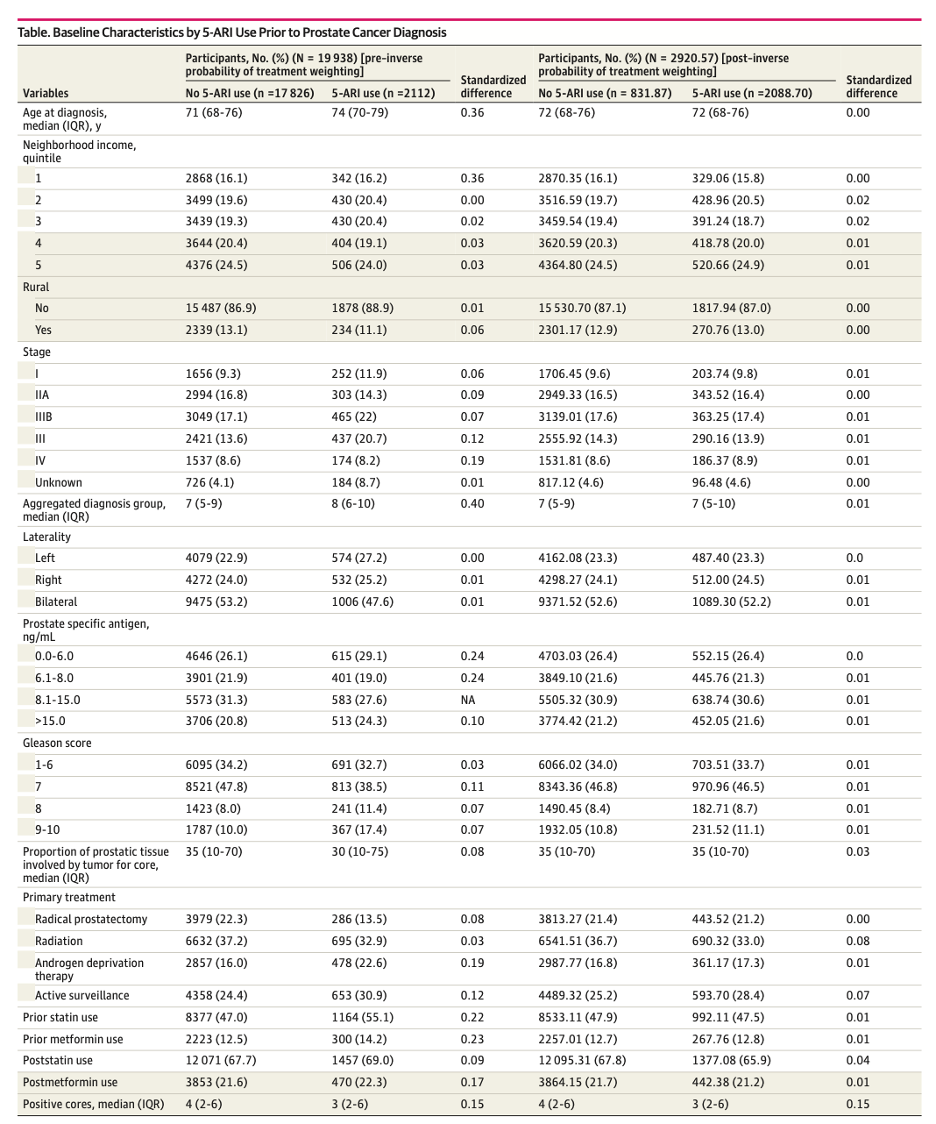
- Age, income quintile, rurality, stage, morphology type, laterality, PSA, total Gleason score, total number of positive cores, maximum percent core, primary treatment, prior statin, post-treatment statin, prior metformin, post-treatment metformin
- Cause-specific hazards models to generate the Hazard ratio (HR) of PCa-mortality in the 5-ARI group vs. the non-user group
- Cumulative incidence function plots to visualize the association between the use of 5-ARIs, overall mortality (OM), and PCa-specific mortality (PCSM)
- We calculated the cumulative incidence of death using attained age as a time scale in 5-ARI users and no users. For the time scale, we assumed the index date (date of PCa diagnosis) as time 0. We used attained age as a time scale and the cumulative incidence of death was calculated at each year of age instead of each year of follow-up thus putting patients with the same age in the risk set together, allowing a non-parametric adjustment for age effect.
- Sensitivity analyses: repeating the primary analysis of 5-ARI use and association with overall and prostate cancer-specific survival using the following restrictions:
- To minimize a potential healthy-user effect we limited the analysis to only those with low comorbidity score (ADG<=5)
- To create a more homogenous cohort in unmeasured characteristics, we used an active comparator approach and included only statin users as a marker of those who actively seek preventive care.
- To minimize bias by indication we limited the analysis to patients who had no history of BPH surgery or urinary retention to enrich the analysis with use of 5-ARI as a primary prevention
- d) To explore whether 5-ARI effects differ based on tumor differentiation and/or advanced stage we ran separate models within strata of Gleason sum ≤6, ≥7, ≥8 and restricted to only those with locally advanced or advanced disease (CS III/IV); e) finally, given that 5-ARIs reduce PSA values by a factor of 0.5, we entered the PSA covariate for the 5-ARI group by multiplying it by two.
- Older
- More comorbidities
- More likely to have had ADT alone or active surveillance as their primary treatment
- More likely to have had a Gleason score of 8 or greater
- More likely to have a lower number of positive cores on average
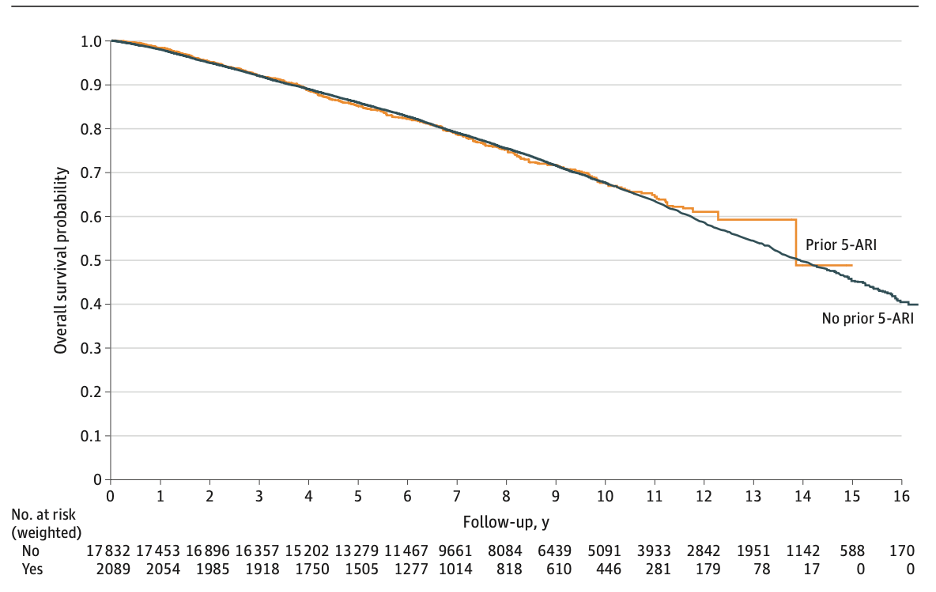
Overall Survival
The median follow-up for the cohort was 8.96 (IQR 6.28-12.17) years, during which 6053 patients (30.4%) died. On crude analysis, 5-ARI use prior to PCa diagnosis was associated with worse overall survival HR 1.24 [95% CI 1.14-1.35, p<0.0001]. However, after IPTW, 5-ARI use was not associated with overall survival on Kaplan-Meier estimates (log-rank P =0.77).
Attained age as a time scale for OS
Age had the biggest effect size on the weight of influence of confounding variables. We explored within strata of age and observed no association of 5-ARI use with OS for patients aged:
- 66 to 75 years (HR, 1.05; 95% CI, 0.92-1.20)
- 76 to 84 years (HR, 1.12; 95% CI, 0.99-1.26)
- 85 years or older (HR, 1.01; 95% CI, 0.81- 1.37)

PCa-Specific Mortality
In crude analyses, like overall survival, 5-ARI use prior to PCa diagnosis was associated with worse PCa-specific mortality HR 1.382 [95% CI 1.14-1.67, p<0.001]. However, after IPTW, 5-ARI use was no longer associated with PCa-specific survival (HR, 1.02; 95% CI, 0.83-1.25; P = .84) as shown below:

We performed a pre-planned subgroup analysis of the subgroups mentioned earlier. Again, we found there continued to be no association of 5-ARI use with overall survival or PCa–specific survival. Of particular importance:
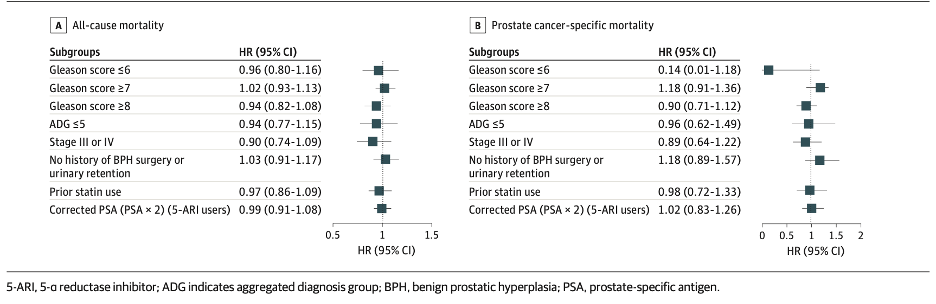
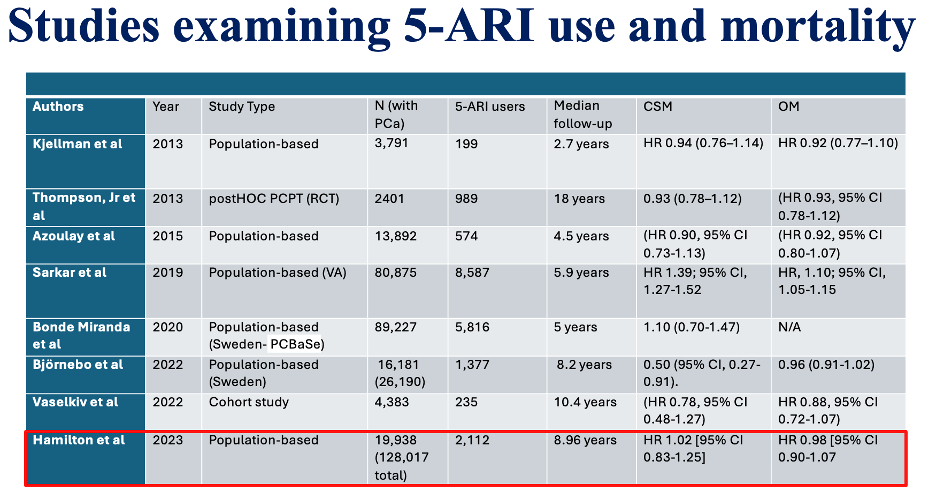
The few studies assessing the association of 5-ARI use before PCa diagnosis with overall and PCa-specific mortality have had mixed results. The table below summarizes the studies, mostly showing results similar to our population-based cohort study, with the exception of a study in the Veterans Affairs database. This study found a higher PCa-specific mortality (HR, 1.39; P <0.001) and all-cause mortality (HR, 1.10; P < 0.001) in 5-ARI users prior to PCa diagnosis. They also observed that pre-diagnostic 5-ARI use was associated with a Gleason score of 8 or greater.12 However, these findings are similar to our crude analyses. Although they adjusted for some population-available variables, they could not adjust for detailed pathological variables as we did, which may explain why we observed no association after IPTW.
Our study is one of the largest, population-based cohorts (n=19,938) with one of the longest follow-up periods (median 8.96 years) reporting PCa-specific mortality. Our detailed demographic, clinical, and especially pathological data collection allows for more accurate covariate adjustment. We found that 5-ARI use prior to PCa diagnosis was not associated with overall or PCa-specific mortality compared with those who did not use 5-ARIs. We believe our results support the safety of 5-ARIs as agents to treat BPH, alopecia, prevent PCa, and support the removal of the FDA label warnings on these products.
Written by:
- Julian Chavarriaga MD, Urologic Oncologist, Cancer Treatment and Research Center (CTIC), Luis Carlos Sarmiento Angulo Foundation via Society of Urologic Oncology (SUO) Fellow, The University of Toronto, Department of Surgical Oncology, Princess Margaret Cancer Centre, Toronto, Ontario, Canada
- Robert J Hamilton MD, MPH, FRCSC, Division of Urology, Department of Surgical Oncology, Princess Margaret Cancer Centre, Toronto, Ontario, Canada.
- Andriole GL, Bostwick DG, Brawley OW, et al: Effect of Dutasteride on the Risk of Prostate Cancer. New Engl J Med 362:1192–1202, 2010
- Klotz L: Words of wisdom. Re: Effect of dutasteride on the risk of prostate cancer. Andriole G, Bostwick D, Brawley O, et al. N Engl J Med 2010;362:1192-202. Eur Urol 58:313, 2010
- Thompson IM, Goodman PJ, Tangen CM, et al: The Influence of Finasteride on the Development of Prostate Cancer. New Engl J Medicine 349:215–224, 2003
- Walsh PC: Chemoprevention of Prostate Cancer. New Engl J Medicine 362:1237–1238, 2010
- Walsh PC, Madden JD, Harrod MJ, et al: Familial Incomplete Male Pseudohermaphroditism, Type 2 — Decreased Dihydrotestosterone Formation in Pseudovaginal Perineoscrotal Hypospadias. New Engl J Medicine 291:944–949, 1974
- Petrow V, Padilla GM, Mukherji S, et al: Endocrine dependence of prostatic cancer upon dihydrotestosterone and not upon testosterone. J Pharm Pharmacol 36:352–353, 1984
- Rittmaster R, Hahn RG, Ray P, et al: Effect of Dutasteride on Intraprostatic Androgen Levels in Men With Benign Prostatic Hyperplasia or Prostate Cancer. Urology 72:808–812, 2008
- Norman RW, Coakes KE, Wright AS, et al: Androgen Metabolism in Men Receiving Finasteride before Prostatectomy. J Urology 150:1736–1739, 1993
- FORTI G, SALERNO R, MONETI G, et al: Three-month treatment with a long-acting gonadotropin-releasing hormone agonist of patients with benign prostatic hyperplasia: effects on tissue androgen concentration, 5 alpha-reductase activity, and androgen receptor content. J Clin Endocrinol Metabolism 68:461–8, 1989
- Musquera M, Fleshner NE, Finelli A, et al: The REDUCE trial: chemoprevention in prostate cancer using a dual 5α-reductase inhibitor, dutasteride. Expert Rev Anticanc 8:1073–1079, 2008
- Hamilton RJ, Kahwati LC, Kinsinger LS: Knowledge and Use of Finasteride for the Prevention of Prostate Cancer. Cancer Epidemiology Prev Biomarkers 19:2164–2171, 2010
- Sarkar RR, Parsons JK, Bryant AK, et al: Association of Treatment With 5α-Reductase Inhibitors With Time to Diagnosis and Mortality in Prostate Cancer. Jama Intern Med 179:812–819, 2019


