The PRAISE-U (PRostate cancer Awareness and Initiative for Screening in the European Union) project emerged from the need for initiatives to support this endeavor.2 Working towards improved early detection of PCa in Europe, our aim was to summarise the existing evidence and highlight areas of consistency and conflict, to present evidence-based foundations for future research, and identify knowledge gaps requiring further investigation.
Being such a widely publicised topic, a substantial body of high-quality research, including systematic reviews (SRs) and meta-analyses (MAs), is already available. Although these SRs and MAs provide valuable insights, they often focus on specific aspects of screening (e.g., decision aids, men’s willingness to be screened, the effectiveness of PSA-based screening trials). This makes it challenging to achieve a comprehensive understanding of the topic. Our aim was to bridge this gap by providing a systematic overview of contemporary evidence on various aspects of the PCa early detection landscape in the EU and the United Kingdom (UK).
Seeking a systematic way to elaborate on contemporary literature while avoiding the repetition of individual studies/trials, we conducted a systematic review including only SRs and/or MAs. To cover multiple aspects, PCa screening/early detection was defined as any step in a ‘healthy’ man’s (e.g., without a history, suspicion, or high risk of PCa) screening pathway, starting at screening invitation and ending at PCa diagnosis (biopsy). Due to the chosen methodology, more recent prospective studies were not included. Hence, the discussion section was used to incorporate these studies and to reflect on all findings, providing context and interpretation.
This SR covers 26 SRs/MAs, which in total included more than 2000 individual studies, spanning from 1990 to 2019. In total, six SRs focused on decision aids (DAs) and educational interventions for PCa screening, two on shared decision-making (SDM), six on men’s perspectives and factors influencing screening decisions, seven on PSA-based screening trials, two on digital rectal examination (DRE), one on PCa screening guidelines, one on risk calculators (RCs), and one focused on PCa epidemiology and risk factors.
In the data, six common themes were identified to present the evidence systematically (see Fig. 1):
- Invitation: Men at general risk should be invited at >50 years (yrs) of age, and testing should be discontinued at >70 yrs or with <10 yrs of life expectancy.
- Decision-making: Most health authorities discourage population-based screening and instead recommend a shared decision-making (SDM) approach. However, implementation of SDM in clinical practice varies widely. Decision aids help men make more informed and value-consistent screening decisions and decrease men’s intention to attempt screening, but they do not affect screening uptake.
- Acceptance: Facilitators for men considering screening include social prompting by partners and clinician recommendations, while barriers include a lack of knowledge, low-risk perception, and masculinity attributes.
- Screening test and algorithm: Prostate-specific antigen (PSA)-based screening reduces PCa-specific mortality and metastatic disease in men aged 55-69 years at randomisation if screened at least twice.
- Harms and benefits: These benefits come at the cost of unnecessary biopsies, overdiagnosis, and subsequent overtreatment.
- Future of screening: Risk-adapted screening, including (pre-biopsy) risk calculators, MRI, and blood- and urine-based biomarkers, could reduce these harms.
To conclude, this review consolidates evidence on PCa screening in the European Union and the UK by thoroughly evaluating the available SRs and MAs on various aspects of the issue. Several key themes have emerged, including invitation policies, shared decision-making strategies, barriers and facilitators for participation, diagnostic tests, and algorithms. While many areas remain contentious and require further investigation, this document, along with other initiatives from the European Association of Urology-led PRAISE-U project, should be viewed as a solid foundation for developing a research framework that could ultimately facilitate the implementation of modern, organized, nationally tailored, and risk-adapted prostate cancer screening programs across Europe and beyond.
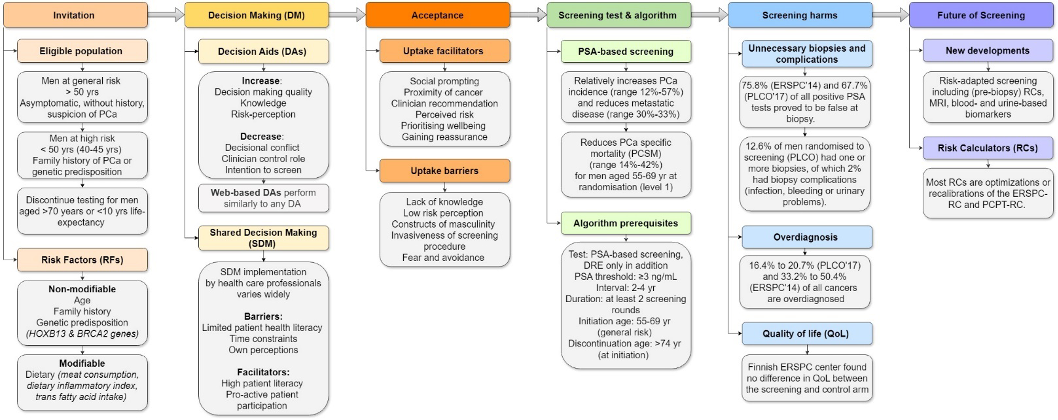
Figure 1. Overview of the identified themes, sub-themes, and main findings
Written by: Renée C.A. Leenen, Monique J. Roobol, & Katharina Beyer
- Erasmus MC Cancer Institute, University Medical Center Rotterdam, Rotterdam, The Netherlands
- European Council. Council updates its recommendation to screen for cancer. Brussels, Belgium: European Council; 2022
- Van Poppel H, Roobol MJ, Chandran A. Early Detection of Prostate Cancer in the European Union: Combining Forces with PRAISE-U. Eur Urol. 2023;84(6):519-22.


