Key eligibility criteria included metastatic prostate adenocarcinoma within 12 weeks of diagnosis, no more than 4 weeks of androgen deprivation therapy (ADT), high-volume PSMA-avid disease on [68Ga]Ga-PSMA-11 PET-CT, and no major discordance on 2-[18F] fluorodeoxyglucose-PET-CT. A total of 130 patients were enrolled at 11 Australian centres between May 2020 and April 2023. A total of 166 patients were registered with 36 (22% ineligible based on baseline PET scans, mainly due to low PSMA uptake. Patients were randomised (1:1) to the experimental arm ([177Lu]Lu-PSMA-617 followed 6 weeks later by docetaxel) or standard-of-care (SOC) arm (docetaxel alone).
The experimental group received two cycles of [177Lu]Lu-PSMA-617 7·5 GBq every 6 weeks intravenously, followed 6 weeks later by six cycles of docetaxel 75 mg/m2 every 3 weeks intravenously, whereas patients in the SOC group received six cycles of docetaxel 75 mg/m2 every 3 weeks intravenously. All patients received in both arms received continuous ADT. The primary endpoint was undetectable prostate-specific antigen (≤0·2 ng/mL) at 48 weeks, with key secondary endpoints including PSA progression-free survival (PSA-PFS), freedom from castration-resistance, radiographic PFS (rPFS), overall survival (OS), quality of life (QoL) and pain, and adverse events (AEs).
A total of 63 patients and 67 patients were enrolled in the experimental and SOC arms, respectively. 4 patients in the SOC arm withdrew before receiving any study treatment. All patients in the experimental arm received both cycles of [177Lu]Lu-PSMA-617. Although dose reductions of docetaxel were more frequent in the experimental compared to SOC arm (33% vs 17%), a high proportion of patients in both arms completed all 6 cycles of docetaxel (79 vs 84%) and discontinuation of docetaxel due to toxicity was also uncommon in both arms (10% vs 6%).
Median follow-up was 2.5 years. Baseline patient characteristics were well matched between treatment arms and are presented in Table 1. Of note, a majority of patients in both arms had multiple poor performance features including high T stage, high-grade group, and high volume disease by conventional imaging.
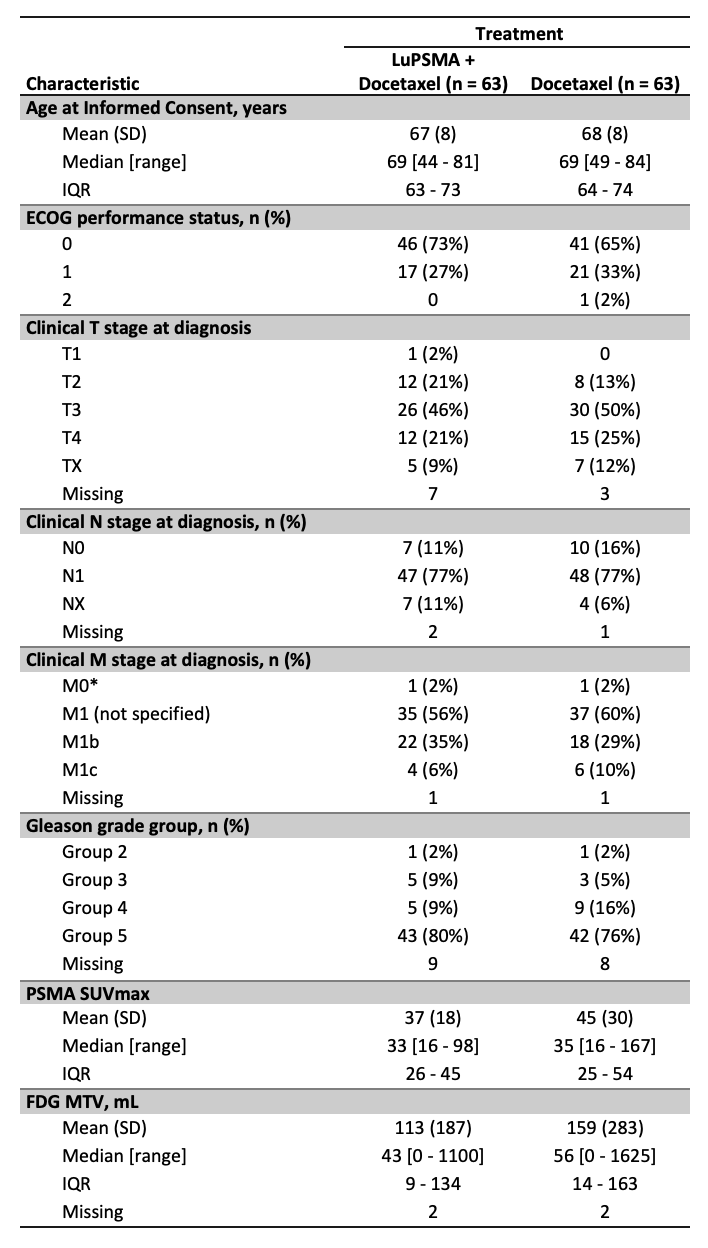
*2 patients who were M0 at diagnosis underwent radical prostatectomy and subsequently relapsed with metastatic disease. Both represent eligibility violations.
The primary endpoint of the study was met (Table 2) with a significant increase in the undetectable PSA rate at 48 weeks favouring the [177Lu]Lu-PSMA-617 plus docetaxel arm. Undetectable PSA rates at any time point also significantly favoured the experimental arm, however, no difference was seen between treatment arms at 12 weeks (Table 2).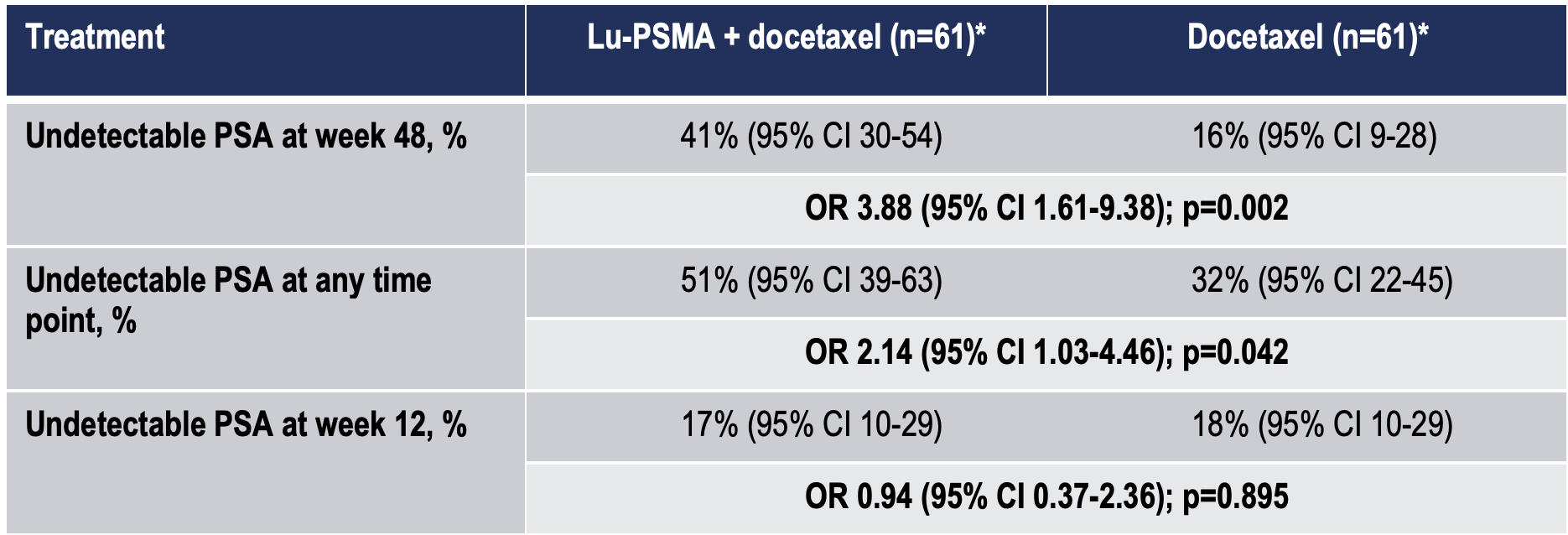
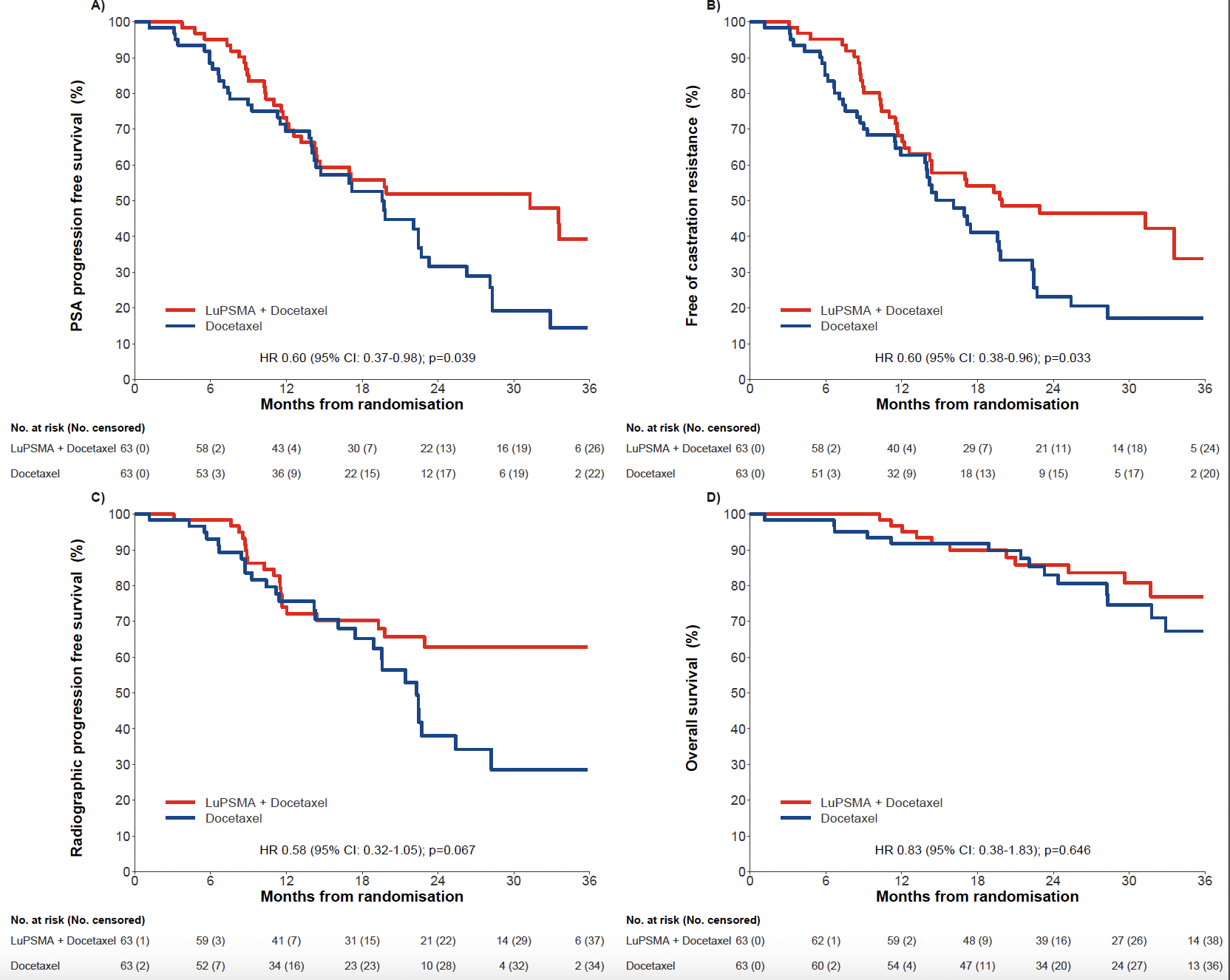
Multiple secondary endpoints were also improved in the [177Lu]Lu-PSMA-617 plus docetaxel arm (Figure 1). Median PSA-PFS and median freedom from castration-resistance were significantly longer in the experimental arm. Median rPFS was also increased in the [177Lu]Lu-PSMA-617 plus the docetaxel arm. OS data were immature with 21% event rate at data cutoff and the median was not reached in either arm (Figure 1).
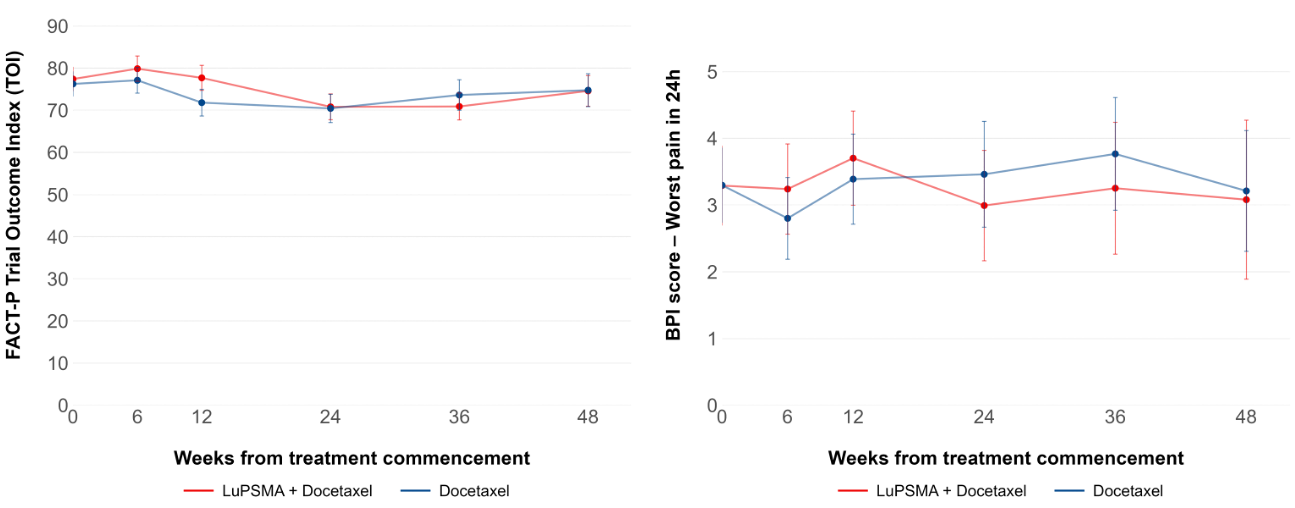
QoL and pain data were comparable between treatment arms, with no significant difference seen at any time point (Figure 2).
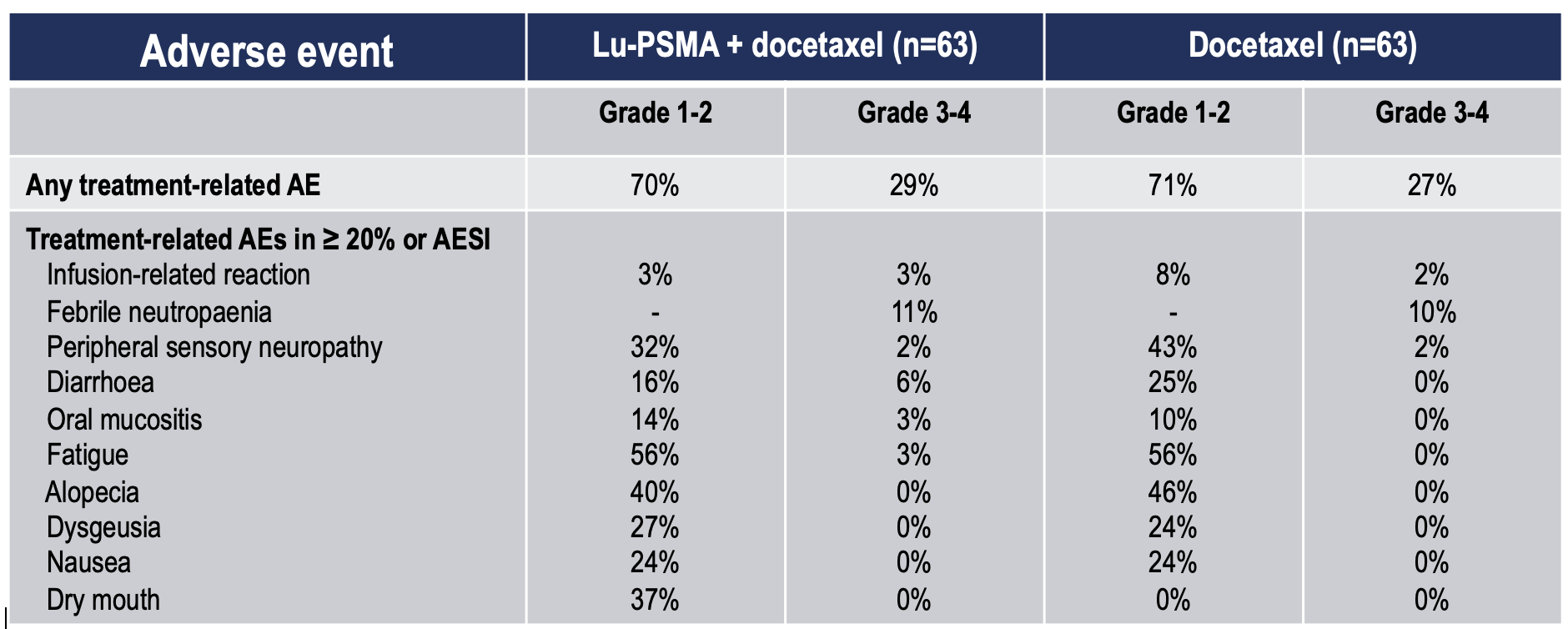
Toxicity was also similar between treatment arms (Table 3) with no increase in Grade 1-2 or Grade 3-4 treatment-related AEs in the [177Lu]Lu-PSMA-617 plus docetaxel arm. Individual AEs were also generally similar with the exception of dry mouth which, as expected, was more common in the [177Lu]Lu-PSMA-617 arm.
The results of UpFrontPSMA are of major significance as it is the first randomised study to show efficacy of [177Lu]Lu-PSMA-617 in mHSPC. The trial was clearly positive with the primary endpoint clearly met with a significant increase in the undetectable PSA rate at 48 weeks. This endpoint was chosen on the basis of prior studies that showed undetectable PSA was associated with improved outcomes in mHSPC patients treated with docetaxel.3,4 Importantly, multiple other secondary endpoints improved in the experimental arm reinforcing the activity of [177Lu]Lu-PSMA-617 plus docetaxel in the trial population. Treatment with [177Lu]Lu-PSMA-617 did not have any detrimental impact on toxicity or on quality of life. These data collectively indicate that [177Lu]Lu-PSMA-617 potentially has a role in the management of mHSPC. It is particularly notable that just 2 cycles of [177Lu]Lu-PSMA-617 had demonstrable efficacy in patients with de novo high-volume mHSPC, a poor prognosis subgroup.
What do these data mean for current and future clinical practice? Since the study commenced, ADT + an androgen receptor (AR) pathway inhibitor (ARPI) +/- docetaxel is now the standard of care for mHSPC. The randomised phase III PSMAddition trial (ClinicalTrials.gov ID NCT04720157) is evaluating ADT + ARPI + [177Lu]Lu-PSMA-617 versus ADT + ARPI with the primary endpoint being rPFS. The study has completed accrual and will further inform on the utility of [177Lu]Lu-PSMA-617 in the context of a contemporaneous SOC arm.
PSMAddition is evaluating the use of 6 cycles [177Lu]Lu-PSMA-617 and this may be an attractive approach for high-volume disease given the activity observed with 2 cycles in the UpFrontPSMA trial population. However, whether 6 cycles of [177Lu]Lu-PSMA-617 are needed for low-volume disease is an open question. Notably, 22% of patients registered for UpFrontPSMA were ineligible based on PET scan criteria, largely due to low PSMA signal in the context of no more than 4 weeks of ADT. With the use of ADT plus an ARPI in PSMAddition, it remains to be seen how beneficial 6 cycles of [177Lu]Lu-PSMA-617 will be for patients with low-volume mHSPC.
Written by: Arun Azad1,2,3 & Michael Hofman2,3
- Department of Medical Oncology, Peter MacCallum Cancer Centre
- Sir Peter MacCallum Department of Oncology, University of Melbourne
- Prostate Cancer Theranostics and Imaging Centre of Excellence, Molecular Imaging and Therapeutic Nuclear Medicine, Peter MacCallum Cancer Centre
- Sartor O, de Bono J, Chi KN, et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med 2021; 385: 1091–103.
- Hofman MS, Emmett L, Sandhu S, et al. [Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet 2021; 397: 797–804.
- Saad F, Hussain MHA, Tombal B, et al. Deep and Durable Prostate-specific Antigen Response to Darolutamide with Androgen Deprivation Therapy and Docetaxel, and Association with Clinical Outcomes for Patients with High- or Low-volume Metastatic Hormone-sensitive Prostate Cancer: Analyses of the Randomized Phase 3 ARASENS Study. Eur Urol 2024; 86: 329–39.
- Harshman LC, Chen Y-H, Liu G, et al. Seven-Month Prostate-Specific Antigen Is Prognostic in Metastatic Hormone-Sensitive Prostate Cancer Treated With Androgen Deprivation With or Without Docetaxel. J Clin Oncol 2018; 36: 376–82.


