Studies have shown that iatrogenic injury to the surrounding neural tissue can cause neuropraxia and increased pro-inflammatory cytokines and reactive oxygen species, which could be driving post-operative outcomes of incontinence and erectile dysfunction.4 Post-operative erectile dysfunction leads to biochemical changes within the penile tissue that promotes apoptosis and fibrosis.5,6 Notable changes include decreased nitric oxide production, decreased cellular production of nitric oxide synthase, decreased concentrations of prostaglandin E1 and prostaglandin E2 which are suppressors of the profibrotic transforming growth factor beta-1 (TGF-β1).7-9
In efforts to combat these molecular-level changes, investigators have started to evaluate perinatal tissue allografts as a potential biologic adjunct to nerve sparing RARP. Perinatal tissue allografts include the umbilical cord, amniotic and chorionic membranes which have favorable growth factors, cytokines, extracellular membranes, anti-inflammatory and anti-fibrotic characteristics which theoretically contribute to their therapeutic benefit in wound healing.10-12 Pre-clinical investigations have revealed several potential cellular mechanisms by which perinatal membrane derived allografts may support nerve repair and regeneration. Common observed benefits of the allografts included decreased formation of neural adhesions, fibrosis, tissue inflammation, and demyelination. Overall, studies suggest that perinatal allografts may have physical and biological characteristics that support healing and regeneration of injured nerves.13-18
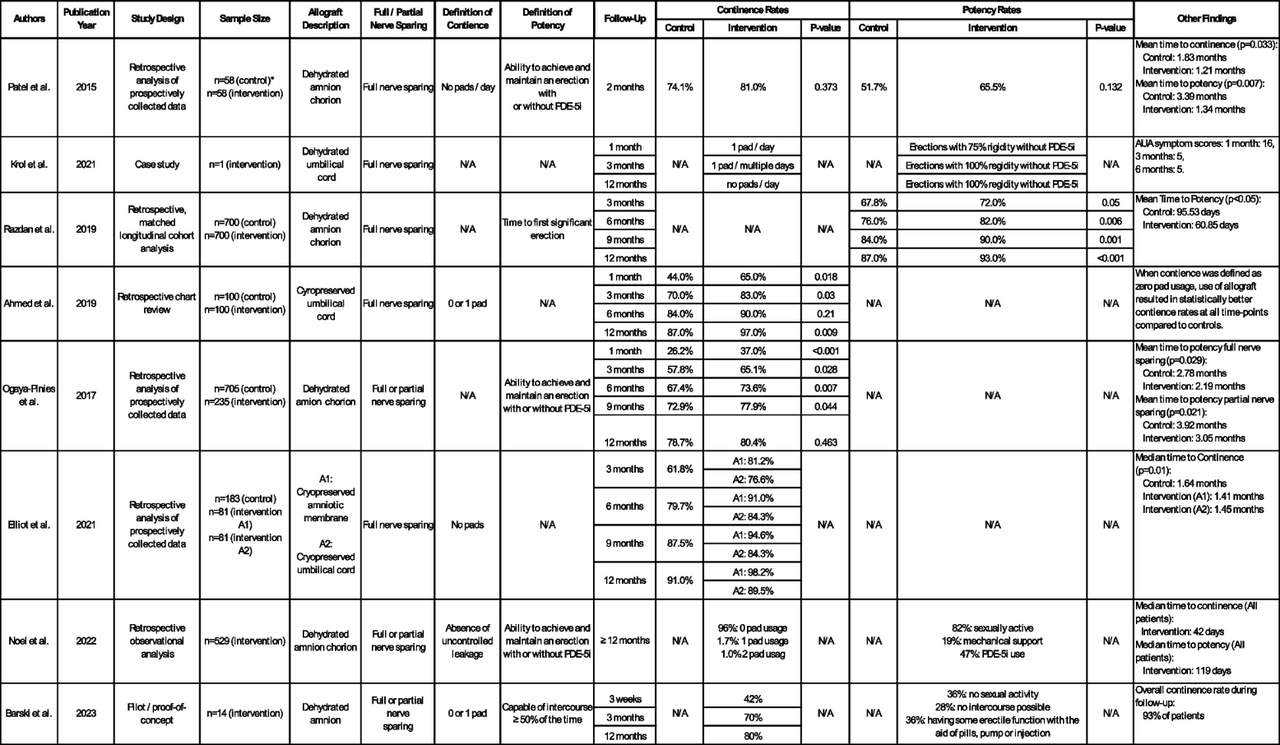
The abstracted manuscript conducted a literature review to evaluate the use of perinatal allografts as nerve wraps in nerve sparing RARP. Eight studies were included for review, six retrospective analyses, one case study, and one pilot proof of concept study.19-26 All evaluations placed a perinatal allograft wrap around the spared neurovascular bundle during RARP. Outcomes included postoperative potency and continence recovery rates. There was variability in the type of allograft used and definitions of achieving continence and potency among the studies. Overall, the evaluated studies consistently demonstrated improved continence rates and time to potency in the allograft vs control group. This evaluation prompts some important questions on the use of perinatal allografts in RARP and the theoretical impact on biochemical recurrence of prostate cancer, potential cost-saving impacts, and the clinical significance of an allografts impact in the hands of an experienced robotic nerve sparing surgeon.
In conclusion, this review of current perinatal tissue allograft use in prostatectomy literature demonstrates that allograft use improves post-operative impotence and incontinence outcomes, which is further strengthened by the underlying biologic plausibility elucidated in basic science research. The emerging evidence has the potential to improve post-prostatectomy outcomes but needs more robust clinical trials to truly prove its safety and efficacy.
Written by: Amanda E Kahn, MD, Mayo Clinic, Jacksonville, Florida
References:
- Siegel Mph RL, Miller KD, Sandeep N, et al. Cancer statistics, 2023. Published online. 2023. https://doi.org/10.3322/caac.21763.
- Cao L, Yang Z, Qi L, Chen M. Robot-assisted and laparoscopic vs open radical prostatectomy in clinically localized prostate cancer: perioperative, functional, and oncological outcomes: A Systematic review and meta-analysis. Med. 2019;98(22). https://doi.org/10.1097/MD.0000000000015770
- Hoyland K, Vasdev N, Abrof A, Bousted G. Post-radical prostatectomy incontinence: etiology and prevention. Rev Urol. 2014;16(4):181–8.
- Ferrini MG, Davila HH, Kovanecz I, Sanchez SP, Gonzalez-Cadavid NF, Rajfer J. Vardenafil prevents fibrosis and loss of corporal smooth muscle that occurs after bilateral cavernosal nerve resection in the rat. Urology. 2006;68(2):429–35. https://doi.org/10.1016/J.UROLOGY.2006.05.011.
- Iacono F, Giannella R, Somma P, Manno G, Fusco F, Mirone V. Histological alterations in cavernous tissue after radical prostatectomy. J Urol. 2005;173(5):1673–6. https://doi.org/10.1097/01.JU.0000154356.76027.4F.
- Casanova MR, Mota P, Vala H, et al. Functional recovery of injured cavernous nerves achieved through endogenous nerve growth factor-containing bioactive fibrous membrane. Acta Biomater. 2023;168:416–28. https://doi.org/10.1016/J.ACTBIO.2023.07.015.
- Gonzalez-Cadavid N, Rajfer J. The pleiotropic effects of inducible nitric oxide synthase (iNOS) on the physiology and pathology of penile erection. Curr Pharm Des. 2005;11(31):4041–6. https://doi.org/10.2174/138161205774913372.
- C B, OO C, KT M, CA P. Nitric Oxide Synthase is Necessary for Normal Urogenital Development. Andrology (Los Angel). 2013;2(01). https://doi.org/10.4172/2167-0250.1000108
- Li K, Zhao J, Wang M, et al. The Roles of Various Prostaglandins in Fibrosis: A Review. Biomol. 2021;11(6). https://doi.org/10.3390/BIOM11060789
- Davies JE, Walker JT, Keating A. Concise Review: Wharton’s Jelly: The Rich, but Enigmatic, Source of Mesenchymal Stromal Cells. Stem Cells Transl Med. 2017;6(7):1620–30. https://doi.org/10.1002/SCTM.16-0492.
- Jirsova K, Jones GLA. Amniotic membrane in ophthalmology: properties, preparation, storage and indications for grafting-a review. Cell Tissue Bank. 2017;18(2):193–204. https://doi.org/10.1007/S10561-017-9618-5.
- Nejad AR, Hamidieh AA, Amirkhani MA, Sisakht MM. Update review on five top clinical applications of human amniotic membrane in regenerative medicine. Placenta. 2021;103:104–19. https://doi.org/10.1016/J.PLACENTA.2020.10.026.
- Ogaya-Pinies G, Palayapalam-Ganapathi H, Rogers T, et al. Can dehydrated human amnion/chorion membrane accelerate the return to potency after a nerve sparing robotic-assisted radical prostatectomy? Propensity score-matched analysis J Robot Surg. 2018;12(2):235–43. https://doi.org/10.1007/s11701-017-0719-8.
- Seif C, Martínez Portillo FJ, Osmonov DK, et al. Methylene blue staining for nerve sparing operative procedures: An animal model. Urology. 2004;63(6):1205–8. https://doi.org/10.1016/j.urology.2003.12.020.
- Stelmashook E V., Voronkov DN, Stavrovskaya A V., et al. Neuroprotective effects of methylene blue in streptozotocin-induced model of Alzheimer’s disease. Brain Res. 2023;1805. https://doi.org/10.1016/J.BRAINRES.2023.148290
- Burgers JK, Nelson RJ, Quinlan DM, Walsh PC. Nerve growth factor, nerve grafts and amniotic membrane grafts restore erectile function in rats. J Urol. 1991;146(2):463–8. https://doi.org/10.1016/S0022-5347(17)37825-4.
- Liu C, Liu D, Zhang X, Hui L, Zhao L. Nanofibrous polycaprolactone/amniotic membrane facilitates peripheral nerve regeneration by promoting macrophage polarization and regulating inflammatory microenvironment. Int Immunopharmacol. 2023;121. https://doi.org/10.1016/J.INTIMP.2023.110507
- Mohammad J, Shenaq J, Rabinovsky E, Shenaq S. Modulation of peripheral nerve regeneration: a tissue-engineering approach. The role of amnion tube nerve conduit across a 1-centimeter nerve gap. Plast Reconstr Surg. 2000;105(2):660–6. https://doi.org/10.1097/00006534-200002000-00027.
- Wolfe EM, Mathis SA, Ovadia SA, Panthaki ZJ. Comparison of Collagen and Human Amniotic Membrane Nerve Wraps and Conduits for Peripheral Nerve Repair in Preclinical Models: A Systematic Review of the Literature. J Reconstr Microsurg. 2023;39(4):245–53. https://doi.org/10.1055/S-0041-1732432.
- Lemke A, Ferguson J, Gross K, et al. Transplantation of human amnion prevents recurring adhesions and ameliorates fibrosis in a rat model of sciatic nerve scarring. Acta Biomater. 2018;66:335–49. https://doi.org/10.1016/J.ACTBIO.2017.11.042.
- Bai J, Liu C, Kong L, Tian S, Yu K, Tian D. Electrospun Polycaprolactone (PCL)-Amnion Nanofibrous Membrane Promotes Nerve Regeneration and Prevents Fibrosis in a Rat Sciatic Nerve Transection Model. Front Surg. 2022;9. https://doi.org/10.3389/FSURG.2022.842540
- Bourgeois M, Loisel F, Obert L, Pluvy I, Gindraux F. Can the amniotic membrane be used to treat peripheral nerve defects? A review of literature. Hand Surg Rehabil. 2019;38(4):223–32. https://doi.org/10.1016/J.HANSUR.2019.05.006.
- Moore MC, Bonvallet PP, Damaraju SM, Modi HN, Gandhi A, McFetridge PS. Biological characterization of dehydrated amniotic membrane allograft: Mechanisms of action and implications for wound care. J Biomed Mater Res B Appl Biomater. 2020;108(8):3076–83. https://doi.org/10.1002/JBM.B.34635.
- Coughlin GD, Yaxley JW, Chambers SK, et al. Robot-assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: 24-month outcomes from a randomised controlled study. Lancet Oncol. 2018;19(8):1051–60. https://doi.org/10.1016/S1470-2045(18)30357-7.
- Sabol TJ, Tran GS, Matuszewski J, Weston WW. Standardized reporting of amnion and amnion/chorion allograft data for wound care. Published online. 2022. https://doi.org/10.1002/hsr2.794.
- Samaritan Biologics - Perinatal Allografts. https://www.samaritanbiologics.com/products/perinatal-allografts. Accessed 21 Apr 2024.


