We conducted a discrete choice experiment (DCE) to understand the preferences of US patients aged >40 years with prostate cancer for different attributes of medical ADT. Six ADT attributes were considered in the DCE: mode and frequency of administration, the need to take a pill to prevent a testosterone surge at initiation of ADT, impact on sexual interest, risk of cardiovascular events, the chance of achieving normal testosterone levels 3 months after ADT discontinuation, and monthly out-of-pocket (OOP) cost.
Participants completed a series of 11 DCE tasks in which they were asked to choose between two hypothetical ADT treatment profiles consisting of varying levels of these six attributes. The survey also collected data on participant sociodemographic characteristics, clinical characteristics, and treatment history. Attribute-level preference weights and attribute relative importance estimates were then derived for groups of respondents with similar treatment preferences, using a latent class analysis. Preference group attribute relative importance estimates were calculated by dividing the range of preference weights for each individual attribute by the sum of the ranges of all attributes and then standardizing them to sum to 100% across all attributes.
Of the 304 participants included, most were aged 65 years and above, and there was a near-even split between participants with and without a history of receiving ADT. Slightly over 50% reported their cancer was confined to the prostate.
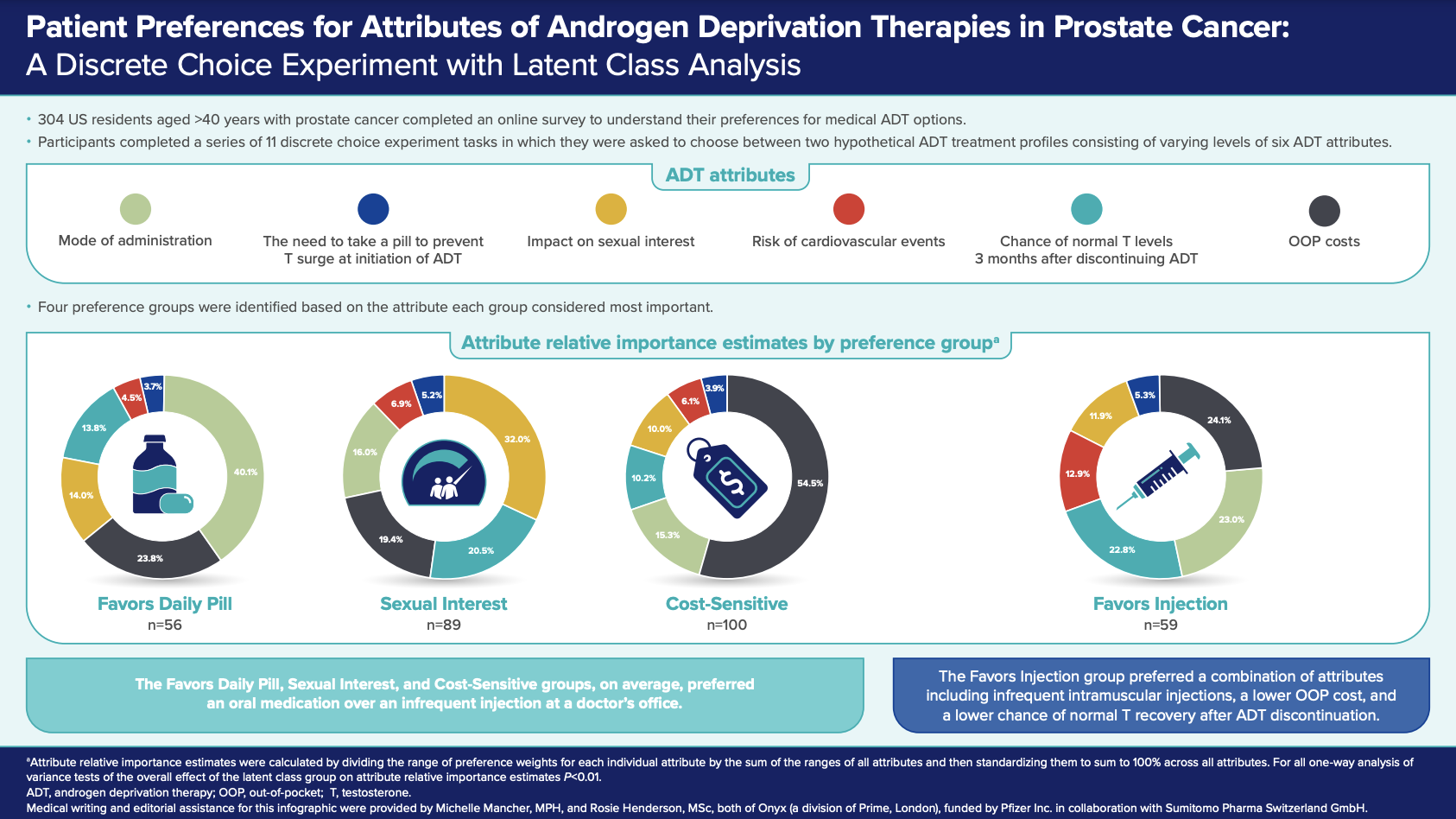
Four preference groups were identified based on the attribute each group considered most important, denoted as Sexual Interest (n=89), Cost-Sensitive (n=100), Favors Daily Pill (n=56), and Favors Injection (n=59). These groups differed in their demographic and clinical characteristics. The Cost-Sensitive group, which included the highest proportion of respondents aged 65 years and above, was most influenced by affordability (relative importance of OOP costs = 54.5%, compared with 19.4–24.1% for the other preference groups). The Favors Daily Pill group, which included the highest proportion of ADT-naïve individuals, was most interested in having the option of oral once-daily treatment (relative importance of mode of administration = 40.1%, compared with 15.3–23.0% for the other preference groups). The Sexual Interest group, which included the highest proportion of respondents aged less than 65 years, favored an option with minimal impact on sexual activity (relative importance = 32.0% compared with 10.0–14.0% for the other preference groups). These three groups generally preferred oral medication over an infrequent injection administered at a doctor’s office. In contrast, the Favors Injection group preferred intramuscular injections every six months at a doctor's office to daily oral administration. Interestingly, this group, which included the highest percentage of ADT-experienced participants, did not have one attribute that was most important but preferred a combination of attributes including infrequent intramuscular injections (relative importance = 23.0%), a lower OOP cost (relative importance = 24.1%), and a lower chance of normal testosterone recovery after ADT discontinuation (relative importance = 22.8%).
This discrete choice analysis underscores the heterogeneity in the priorities and preferences of patients with prostate cancer. Patients will generally consider their disease and personal situation to prioritize factors related to administration, side effects, potential impact on sexual activity, or cost when choosing ADT treatment. Understanding and discussing these preferences may help healthcare providers tailor treatment plans to individual patient needs through shared decision-making. This locus of control can potentially improve long-term adherence to ADT and patient satisfaction. Future research should continue to explore this area to further our understanding of patient preferences and their impact on treatment outcomes.
Disclosures
Sean P. Collins is a consultant for Boston Scientific Corporation, Sumitomo Pharma America, Inc. (formerly Myovant Sciences Inc.), and Pfizer Inc.; and has received honoraria and research funding from Accuray Inc.
Writing support for this review was provided by Michelle Mancher, MPH, and editorial support was provided by Rosie Henderson, MSc, both of Onyx (a division of Prime, London, UK) and funded by Pfizer Inc. in collaboration with Sumitomo Pharma Switzerland GmbH. Pfizer’s generative artificial intelligence (AI) assisted technology MAIA (Medical Artificial Intelligence Assistant) was used to develop an initial draft of this commentary. After using this tool, the author reviewed and edited the content as needed and takes full responsibility for the final work.
Written by: Sean P. Collins, MD, PhD, Vice Chair of Faculty Affairs, Department of Radiation Oncology, University of South Florida, Tampa, FL
Reference:
- Hauber B, Hong A, Hunsche E, Maculaitis MC, Collins SP. Patient Preferences for Attributes of Androgen Deprivation Therapies in Prostate Cancer: A Discrete Choice Experiment with Latent Class Analysis. Adv Ther. 2024;41(10):3934–3950. doi: 10.1007/s12325-024-02955-1. Epub 2024 Aug 21. PMID: 39167332; PMCID: PMC11399292.
- Lowrance W, Dreicer R, Jarrard DF, Scarpato KR, Kim SK, Kirkby E, Buckley DI, Griffin JC, Cookson MS. Updates to Advanced Prostate Cancer: AUA/SUO Guideline (2023). J Urol. 2023;209:1082–1090.
- Crawford ED, Heidenreich A, Lawrentschuk N, Tombal B, Pompeo ACL, Mendoza-Valdes A, Miller K, Debruyne FMJ, Klotz L. Androgen-targeted therapy in men with prostate cancer: evolving practice and future considerations. Prostate Cancer Prostatic Dis. 2019;22:24–38.
- Raja T, Sud R, Addla S, Sarkar KK, Sridhar PS, Talreja V, Jain M, Patil K. Gonadotropin-releasing hormone agonists in prostate cancer: A comparative review of efficacy and safety. Indian J Cancer. 2022;59:S142–S159.
- U.S. Food & Drug Administration. Prescribing information: ORGOVYX (relugolix) tablets, for oral use. 2020. [Accessed May 16, 2024].


