Patients reaching this PSA threshold experience significantly lower rates of disease progression, emphasizing the value of PSA response as a prognostic factor. Notably, patients with incomplete PSA responses face a substantially higher risk, with nearly 50% experiencing disease progression within 24 months.
The findings presented here could guide the development of personalized treatment algorithms based on individual PSA response, informing the design of tailored follow-up strategies that incorporate both imaging and PSA monitoring. Patients with suboptimal PSA responses to ARPIs may benefit from intensified or modified therapeutic approaches. In contrast, those demonstrating a favorable PSA response might be suitable candidates for reduced-intensity therapies or intermittent treatment regimens.
Our study underscores the utility of the three-month PSA response as a valuable predictor of rPFS and potentially overall survival. Additional research is necessary to explore the clinical implications of PSA response in refining treatment decisions and patient management, especially to identify subgroups that may benefit from therapy de-escalation or more intensive follow-up.
Several ongoing studies aim to address these questions. To test the hypothesis that patients with a complete PSA response (<0.02 ng/mL) may be candidates for therapeutic de-escalation, it would be ideal to compare long-term oncological outcomes in patients who reduce or discontinue androgen therapy with a control group maintaining full androgen blockade. Such study designs, involving long-term follow-up for both groups, would provide critical insights into the feasibility of safely discontinuing therapy—potentially reducing costs and adverse effects without compromising outcomes.
Finally, a subgroup analysis, not included in the original manuscript, reveals no significant association between synchronous versus metachronous metastasis timing across the three PSA response groups and rPFS. This finding supports the predictive utility of the three-month PSA response for rPFS, regardless of metastasis timing.
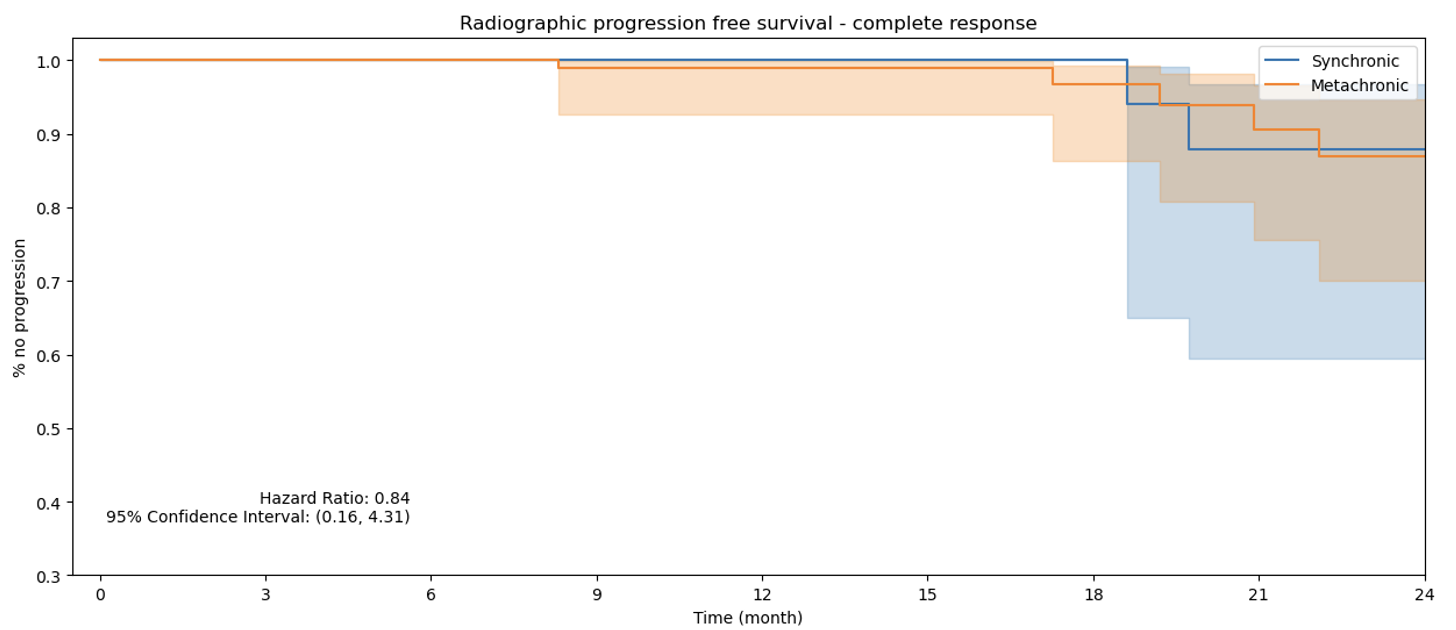
Figure 1. rPFS Complete Response PSA Group.

Figure 2. rPFS Partial Response PSA Group.
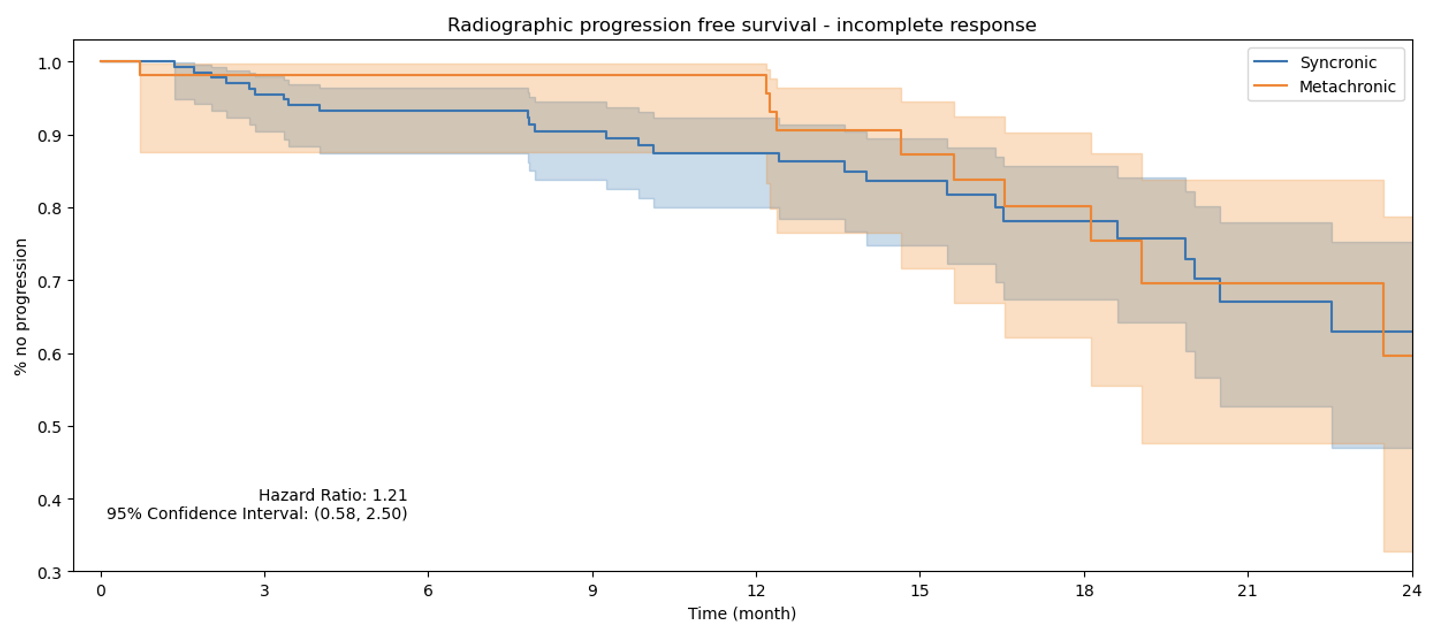
Figure 3. rPFS Incomplete Response PSA Group.
Written by: Mario Hassi, Department of Urology, Hospital DIPRECA, Santiago, Chile
Read the Abstract


