Alterations in the tumor suppressor genes (TSGs) RB1, PTEN, and TP53 are associated with treatment resistance, worse survival, and aggressive variants of prostate cancer (AVPC). We previously developed and validated a signature reflecting low TSG expression (TSGlow) that was associated with poor outcomes in patients with mHSPC treated with ADT ± docetaxel. The signature was considered TSGlow when 2 out of these 3 TSGs presented low expression measured with nCounter (Nanostring), and TSGwt in the remaining cases.
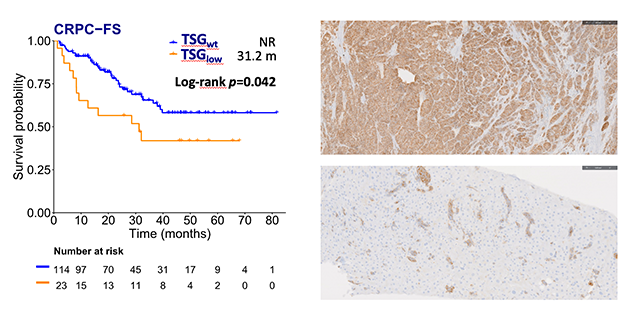
In this multicenter biomarker study, enrolling patients from different Spanish hospitals, we further validated the TSGlow signature in mHSPC patients treated with ADT+ARPI. Of the 137 patients included (35 were treated with ADT + enzalutamide, 99 with ADT + abiraterone, and 4 with ADT + apalutamide), 77.4% had de novo stage IV cancer and 44.5% had high-risk disease. TSGlow (16.8%) was correlated with visceral metastases (p=0.013), as well as high-risk disease (p=0.038), and higher Gleason score (p=0.026). Regarding the clinical outcomes, low TSG expression was associated with a shorter castration-resistant prostate cancer (CRPC) free survival (CRPC-FS)(hazard ratio [HR] 1.9; p=0.046). Specifically, patients with low expression of both RB1 and PTEN had significantly shorter CRPC-FS (HR 2.7; p=0.011) and overall survival (HR 2.5; p=0.017).
We further explored if patients who progressed to CRPC presented aggressive variants of prostate cancer (AVPC), defined with Aparicio’s clinical criteria. We found that patients harboring TSGlow were more likely to develop AVPC during CRPC progression (66.7% vs 18.7%, p=0.01). All patients considered PTENlow-RB1low presented AVPC criteria at CRPC. Besides, in 4 patients, a biopsy was performed at the time of CRPC and all of them showed neuroendocrine differentiation.
These findings suggest that the TSGlow signature could serve as a useful prognostic biomarker to predict tumor aggressiveness and identify patients at higher risk for poor clinical outcomes, guiding treatment decisions and monitoring strategies. However, further prospective studies are needed to validate our findings and refine therapeutic approaches for patients with this aggressive subset of prostate cancer.
Written by: Marta Garcia de Herreros1,2,3 & Natalia Jimenez1
- Translational Genomics and Targeted Therapeutics in Solid Tumours Lab, Fundació de Recerca Clínic Barcelona-Institut d'Investigacions Biomèdiques August Pi i Sunyer, Barcelona, Spain.
- Medical Oncology Department, Hospital Clínic, Barcelona, Spain.
- Uro-Oncology Unit, Hospital Clínic, University of Barcelona, Barcelona, Spain.


