During these last ten years we have learned several things: First, targeted agents improve objective response rates (ORR), progression-free survival (PFS), and very likely overall survival (OS). Second, checkpoint inhibitors (ICI) work well in mRCC patients including as 2nd-3rd line therapy, as well as the 1st line. Third, the ICI combination of ipilimumab + nivolumab works even better, with an OS benefit when this combination is used as first-line therapy. Due to the impressive results, this combination has shown, it has been defined as the standard of care in intermediate and high-risk mRCC. Fourth, VEGF tyrosine kinase inhibitor (TKI) might have immunomodulatory effects, resulting in the combination of VEGFR TKI with ICI to cause a very high response rate.
The first study discussed is the JAVELIN Renal 101: A randomized phase 3 trial of Avelumab + Axitinib vs. Sunitinib as first-line treatment of advanced renal cell carcinoma1. The study design of JAVELIN Renal 101 is shown in figure 1. The primary endpoint was PFS.
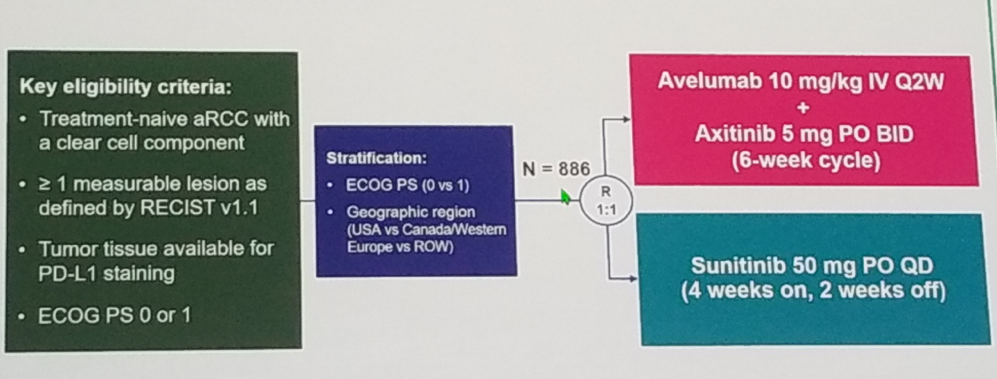
Figure 1 – JAVELIN 101 study design:
The results demonstrate an advantage in median PFS favoring the avelumab + axitinib arm in the PD-L1 positive patients (13.8 vs. 7.2 months, HR 0.61 [95% CI o.475-0.79], p<0.0001), as can be seen in figure 2. The PFS remained better in the combination group, in all the various subgroups analyses.
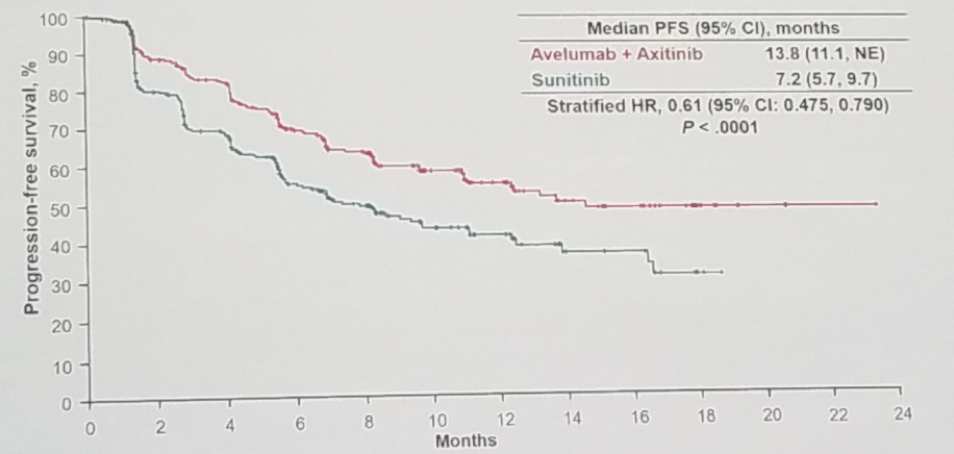
Figure 2 – Progression-free survival in the PD-L1 positive group in the JAVELIN 101 study:
The objective response rate was also much better in the combination arm in all patients and PD-L1 positive patients (51% vs. 26%, and 55% vs. 26%, respectively). The OS data were still immature after a follow-up of only 24 months. 14% of the patients in the combination arm had an event, white 17% of the patients in the sunitinib arm had an event. The median survival had not been reached by either group. The response rates reported in the combination arm are the highest ever reported when compared to previous studies assessing various drugs in the setting of mRCC (Figure 3).
The current ESMO guidelines for 2018 recommendations for first-line treatment in clear cell mRCC are the following:
- - Good risk – Sunitinib/pazopanib/bevacizumab/tivozanib
- - Intermediate risk – Nivolumab and Ipilimumab
- - Poor risk – Nivolumab and Ipilimumab

Figure 3 – Response rates in front line metastatic RCC trials:
The second study presented was the: Molecular correlates differentiates response to atezolizumab + bevacizumab vs. sunitinib: results from a phase 3 study (IMmotion 151) in untreated metastatic renal cell carcinoma.2 The IMmotion 151 study included mRCC treatment naïve patients with clear cell and sarcomatoid histology. Patients were randomized to either sunitinib or the combination of atezolizumab and bevacizumab. The co-primary endpoints were PFS in PD-L1 positive tumors, and OS in intention to treat analysis. The study design is shown in figure 4.
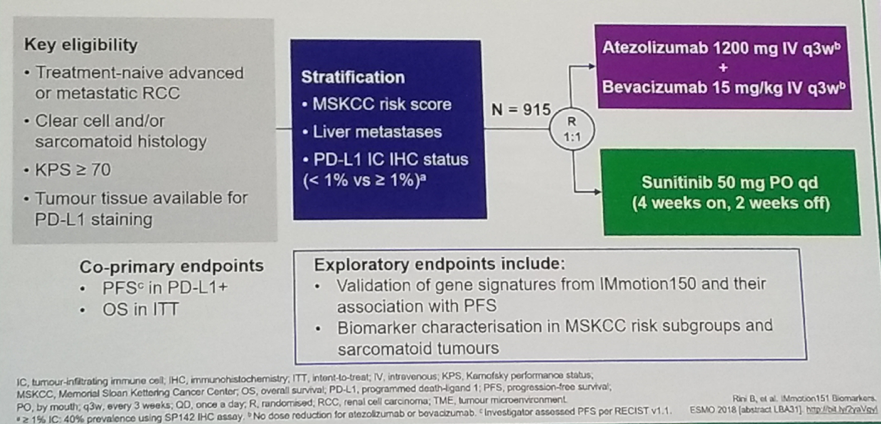
Figure 4 – Immotion 151 study design:
The IMmotion 150 study, presented in ESMO 2018, demonstrated that the relative expression of angiogenesis and T-effector gene signatures identified differential PFS benefits for the different treatment groups. Sunitinib showed improved PFS in angiogenesis high vs. angiogenesis low subsets. Atezolizumab +bevacizumab improved PFS in T effector high and angiogenesis low tumors.
Summarizing the results, the pre-specified analyses in IMotion 151 validated angiogenesis and T effector gene signatures identified in IMmotion 150. The favorable risk patients are characterized by predominant angiogenesis high gene signature. Sarcomatoid RCC is characterized by an Angiogenesis low gene signature, a T-effector high gene signature, and a higher PD-L1 expression, making this entity highly responsive to treatment with atezolizumab + bevacizumab. The results of this study help inform future strategies and enable us to personalize therapy according to the various risk groups.
Next, Dr. Bono discussed some of the more interesting studies in bladder cancer, presented in ESMO 2018. In recent years, there have been five new systemic immunotherapeutic agents that have been approved. These drugs are being tested in various settings of bladder cancer and other cancers as well.
The two studies that were discussed include:
- Checkmate 032 – Nivolumab alone or in combination with Ipilimumab in patients with platinum-pretreated metastatic urothelial carcinoma.3
- Interim analysis of Keynote-057 – Pembrolizumab for high-risk non-muscle invasive bladder cancer (NMIBC) unresponsive to Bacillus Calmette-Guerin (BCG).4
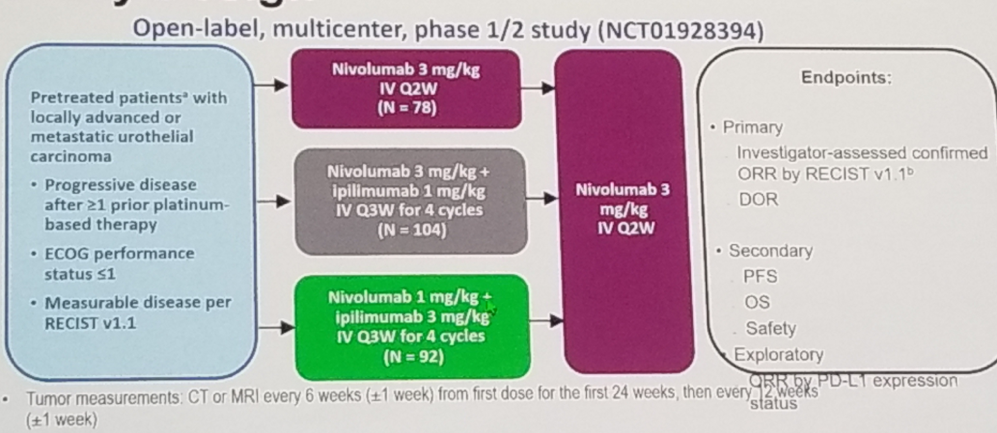
Figure 5 – Checkmate 032 study design:
The last study discussed was the Pembrolizumab in the NMIBC setting (Keynote 057 trial). The study design is demonstrated in Figure 6. In this trial patients with NMIBC who were unresponsive to BCG therapy were given pembrolizumab and evaluated with cystoscopy, cytology and CT scans at regular intervals.
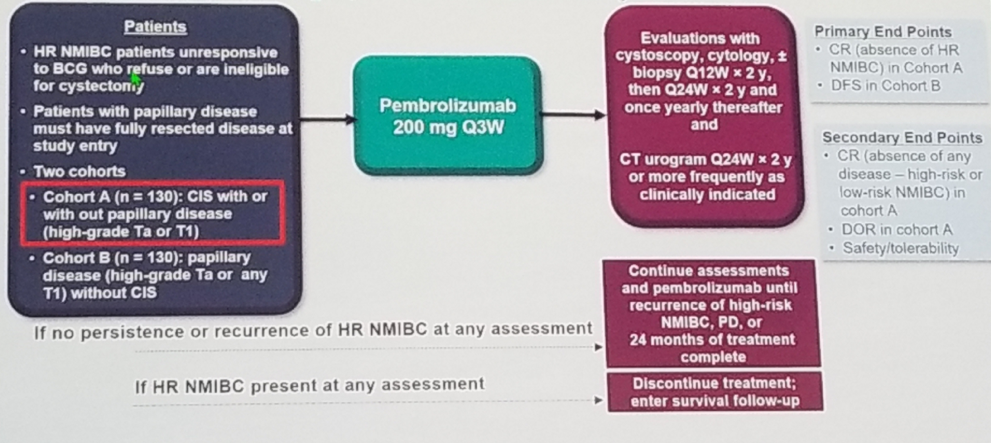
Figure 6 – Keynote-057 Study design:
The results of this trial were quite extraordinary, showing a complete response rate of 38.8% because of pembrolizumab therapy. The median duration of the response was not reached after 14 months of follow-up, with more than 80% of the patients demonstrating a complete response six months after treatment.
Summarizing the results of all these trials, there is uncertainty in the frontline setting with cis-ineligible patients treated with ICI. This therapy should not be recommended for patients with negative PD-L1 (<1%). The ICI combination looks promising, but phase 3 results are still needed. Lastly, Pembrolizumab has demonstrated significant activity in the NMIBC disease and remains to be further studied in this setting (Keynote 676, phase 3 trial).
Presented by: Petri Bono, The University of Helsinki, Helsinki, Finland
References:
1. Motzer RJ et al. ESMO 2018
2. Rini BI et al. EMSO 2018
3. Rosenberg JE et al. ESMO 2018
4. De Wit RG et al. ESMO 2018


