ALEXANDRIA, VA USA (Press Release) - February 15, 2011
New studies on the screening and treatment of genitourinary cancers were released today in advance of the fourth annual Genitourinary Cancers Symposium, being held February 17-19, 2011, at the Orlando World Center Marriott in Orlando, Florida.
The results of three studies were highlighted in a media presscast (press briefing via live webcast):
- Large screening study shows reduced risk of prostate cancer death for men with low initial PSAs: A large prostate cancer screening study of middle-aged and elderly men showed that an initial Prostate-Specific Antigen (PSA) score of 3.0 ng/ml appears to be an appropriate minimum cut-off level to determine the need for biopsy. Few men in the study with low first-time PSAs below 3.0 developed prostate cancer and died from the disease, and the findings may help better target testing for those at risk.
- Proficiency in robotic-assisted prostate surgery requires experienced specialists: In a study to determine the surgical learning curve for robotic-assisted laparoscopic radical prostatectomy (RALP) operations, a retrospective analysis of the results of nearly 3,800 procedures showed that it took more than 1,600 prostate cancer surgeries for surgeons to become proficient at the RALP procedure and be able to remove the cancerous prostate consistently with its edges clear of cancer.
- Dutasteride helps slow early-stage prostate cancer growth: A new study has shown that a drug commonly used to treat men with an enlarged prostate gland - dutasteride (Avodart) - may also slow the growth of early-stage prostate cancer among men participating in "active surveillance" of their disease.
"While the use of PSA in determining which men should have biopsies for suspected prostate cancer has sometimes been controversial, the results of a study presented today provide important new insights on the value of PSA in helping practitioners make treatment decisions," said Nicholas J. Vogelzang, MD, Chair and Medical Director of the Developmental Therapeutics Committee of US Oncology, who moderated today's presscast. "Other significant studies presented today show the potential of an already commonly used prostate drug to slow cancer growth. And a second evaluates the expertise required to carry out often difficult surgical procedures."
Genitourinary cancers include those of the prostate, kidney, bladder and testis, as well as less common cancers such as those of the penis, ureters and other urinary organs. In 2010, more than 358,000 people in the United States were diagnosed with genitourinary cancers and more than 61,000 died of these diseases. The most common genitourinary cancer is prostate cancer, which was diagnosed in nearly 218,000 men in the United States in 2010 and claimed more than 32,000 lives.*
The Genitourinary Cancers Symposium is co-sponsored by the American Society of Clinical Oncology (ASCO), the American Society for Radiation Oncology (ASTRO) and the Society of Urologic Oncology (SUO).
More information for media: www.asco.org/GUpresskit11
Relevant Links on Cancer.Net, the oncologist-approved cancer information website from the American Society of Clinical Oncology.
- 2011 GU Cancers Symposium Cancer Advances
- 2011 GU Cancers Symposium Podcast
- Cancer.Net Guide to Prostate Cancer
- ASCO Answers - Prostate Cancer
- ASCO Answers - Prostate Cancer (Spanish)
- Cancer Screening
- Talking With the Doctor About PSA Screening
- Understanding Cancer Surgery
- Basics of Cancer Surgery: Video with Robert Sticca, MD
*American Cancer Society, Cancer Facts and Figures 2010
Lead Author: Meelan Bul, MD
Erasmus University Medical Center
Rotterdam, The Netherlands
Oral Abstract Session
Thursday, February 17, 2011
1 PM-2:50 PM ET
A large prostate cancer screening study of middle-aged and elderly men that included repeat visits showed that an initial Prostate-Specific Antigen (PSA) score of 3.0 ng/ml appears to be an appropriate minimum cut-off level to determine the need for biopsy. Few men in the study with low first-time PSAs below 3.0 developed prostate cancer and died from the disease. Researchers also found that within this group of low-risk men, the higher the initial PSA, the greater the risk of developing prostate cancer and more aggressive disease, and of dying from prostate cancer.
"We now know more about prostate cancer detected by PSA screening in men with initial PSA scores of less than 3.0," said lead author Meelan Bul, MD, PhD candidate, Department of Urology at Erasmus University Medical Center in Rotterdam, The Netherlands. "Our results strengthen the justification of the use of PSA in risk stratification for screening purposes. This means that we can possibly avoid unnecessary testing, diagnosis and treatment of less aggressive disease, with the accompanying side-effects, by focusing biopsies and other follow-up on men with higher initial PSAs above 3.0." She noted that while PSA level by itself doesn't necessarily enable doctors to distinguish between cancer and benign conditions, the results are used to help decide if further testing for prostate cancer is warranted.
In the study - part of the larger European Randomized Study of Screening for Prostate Cancer - researchers analyzed both incidence of and deaths from prostate cancer among 42,376 men between ages 55 and 74 living in the Rotterdam area. Participants in the population-based study were randomized to either screening or a control arm. Of the 42,376 men, 19,950 were initially screened and biopsies were recommended for those with PSA scores of 3.0 or above only, with four-year screening intervals.
Researchers were interested in the number of men with an initial PSA value of less than 3.0 who would be diagnosed with prostate cancer and the number of men who would die of this disease during long-term follow-up. They found 15,758 (79 percent) of the men had an initial PSA under 3.0. Between 1993 and 2008, 915 of those men were diagnosed with prostate cancer - with a median follow up of 11 years - with only 23 deaths. Of the 915 diagnosed, 182 were detected between screenings, often indicating a faster-moving disease, and overall, 169 (1.1 percent) were determined to be aggressive prostate cancers.
Overall, prostate cancer incidence and deaths increased significantly with higher PSA levels. Only 129 men (1.8 percent) of 7,126 men with PSA scores below 1.0 were eventually diagnosed with prostate cancer, with only three deaths (.04 percent). Of the 6,156 men with PSA scores between 1.0 and 1.9, 415 (6.7 percent) developed prostate cancer, with 11 deaths (.18 percent). The researchers found 2,476 men with PSA levels between 2.0 and 2.9, with 371 cases of prostate cancer (15.7 percent) and nine deaths (.36 percent).
"The 3.0 score appears to be an appropriate threshold for the study because approximately 80 percent of the men ages 55 to 74 years had a PSA under 3.0, with few deaths from prostate cancer. At the same time, we still found a group of men with aggressive prostate cancer and we need improved methods of detecting aggressive disease. These results can contribute to better individual management of men in PSA-based screening programs," said senior investigator Monique Roobol, PhD, an epidemiologist in the Department of Urology at Erasmus University Medical Center.
The investigators suggested that future research focus on improving the detection of aggressive prostate cancers, including better risk stratification methods and new molecular and genetic markers.
Prostate cancer incidence and disease-specific survival in men participating in the ERSPC with an initial PSA less than 3.0 ng/mL.
Authors: M. Bul, P. J. van Leeuwen, X. Zhu, F. H. Schröder, M. J. Roobol; Erasmus Medical Centre, Rotterdam, Netherlands
Background: The European Randomized Study of Screening for Prostate Cancer (ERSPC) applies a prostate-specific antigen (PSA) cut-off >3.0 ng/mL as an indication for biopsy. We analyzed the incidence and disease-specific mortality for prostate cancer (PC) within ERSPC Rotterdam for men with an initial PSA <3.0 ng/ml in a 15-year follow-up period.
Methods: From 1993-1999, a total of 42,376 men identified from population registries in the Rotterdam region (55-74 yrs) were randomized to a screening or control arm. During the first screening round 19,950 men were screened, with biopsies being initially recommended in case of abnormal DRE or PSA >4.0 ng/mL. From 1997 on, solely PSA >3.0 ng/mL was used. The screening interval was 4 yrs. A total of 15,758 men (79%) had an initial PSA <3.0 ng/mL. Follow-up was complete until January 2009.
Results: From 1993-2008, 915 PC cases were diagnosed in 15,758 men (5.8%, median age 62.3 yrs) with an initial PSA <3.0 ng/mL (733 screen detected and 182 interval detected). Median follow-up was 11 yrs. PC incidence increased significantly with higher initial PSA levels (table). Aggressive PC (clinical stage >T2c, Gleason score >8, PSA >20 ng/mL, positive lymph nodes or metastases at diagnosis) was detected in 65/733 screen detected PC (8.9%) and 102/182 interval detected PC (56.0%). PC death occurred in 23 cases (5 screen detected and 18 interval detected) in the total population (0.15%), with increasing risk in men with higher initial PSA values.
Conclusions: The risk of (aggressive) PC and PC mortality in a screening population with initial PSA <3.0 ng/mL increases significantly with higher PSA levels. The risk of dying of PC is minor in men with initial PSA <1.0 ng/mL. Interval detected PC is more aggressive and has a substantial influence on PC specific mortality.
Disclosures: Fritz H. Schroder: Consultant or Advisory Role with GlaxoSmithKline; Monique J. Roobol: Consultant or Advisory Role with Bechman Coulter, GlaxoSmithKline and Gen-Probe.

Lead Author: Prasanna Sooriakumaran, MD, PhD
Cornell University
New York, NY
General Poster Session A
Thursday, February 17, 2011
11:45 AM-1:15 PM ET
In a study to determine the surgical learning curve for robotic-assisted laparoscopic radical prostatectomy (RALP) operations, a retrospective analysis of the results of nearly 3,800 procedures showed that it took more than 1,600 prostate cancer surgeries for surgeons to become proficient at the RALP procedure and be able to remove the cancerous prostate consistently with its edges clear of cancer.
RALP is a relatively new technology that has several advantages over typical laparoscopic surgery, which uses awkward "chopstick-like" instruments. RALP provides surgeons with 3-dimensional vision, improved magnification, hand tremor filtering, and a range of motion similar to the human wrist.
"The robotic platform has been shown to take less training time to learn to safely perform prostate cancer surgery compared to its open and laparoscopic surgery counterparts, but we see that becoming expert at the robotic operation takes much longer than just simply developing a base level of competence," said lead author Prasanna Sooriakumaran, MD, PhD, a visiting fellow in urology at the Weill Cornell Medical College in New York. "This research shows that optimizing patient outcomes in terms of positive margin rates takes much more experience. In this regard the operation is more difficult than previously thought."
When surgeons remove a cancerous prostate, they want to be sure the edges of the prostate are clear of cancer. A cancer-free edge, or margin, is called a negative surgical margin. A positive surgical margin (PSM) suggests that some cancer still remains in the body, and studies have shown that patients with positive surgical margins are more likely to recur.
Dr. Sooriakumaran and his colleagues reviewed the surgical results of 3,794 patients who underwent RALP over a six-year period between 2003 and 2009 in procedures performed by three surgeons from the University of Pennsylvania, Karolinska Institute and Cornell University. The researchers determined mean overall PSM rates and operation lengths for each surgeon at intervals of every 50 operations. The investigators found that the PSM rates for all patients continued to improve with increasing surgeon experience. It took more than 1,600 cases to achieve a PSM rate of less than 10 percent, which is considered a standard goal for such surgeries.
"Even for those who do hundreds of cases per year, it takes a long time to get to the stage where they are getting the best possible cancer control results," Dr. Sooriakumaran said. "Our results show that it is possible to get good cancer cure rates and low surgical margins with this operation, but it takes a significant amount of experience."
The investigators recommended that this type of radical prostatectomy procedure be performed by surgeons who see large volumes of patients.
A multi-institutional study of 3,794 patients undergoing robotic-assisted laparoscopic radical prostatectomy to determine the surgical learning curve for positive margins and operating time.
Authors: P. Sooriakumaran, M. John, R. Leung, D. Peters, D. Lee, P. Wiklund, A. Tewari; Weill Cornell Medical College, New York, NY; University of Pennsylvania, Philadelphia, PA; Karolinska Institute, Stockholm, Sweden
Background: The surgical learning curve for robotic assisted laparoscopic radical prostatectomy (RALP) is often cited as being shorter than for other surgical modalities. However, while this appears true with regards to surgical safety, the learning curve for more refined variables like positive surgical margin (PSM) rate and operative time (OT) is not well established. Our objective was to assess the surgical learning curve for RALP in terms of these parameters.
Methods: We performed a retrospective cohort study of 3,794 patients who underwent RALP between Jan 2003 and Sep 2009 by three surgeons (DL, PW, AKT) from three centers (UPenn, Karolinska, Cornell). Mean overall PSM rates and mean overall OT were calculated for all three surgeons at intervals of 50 RALPs per surgeon, and learning curves for these means were fit using a loess method. R version 2.71 was used for all statistical analysis.
Results: The learning curve for PSM rates for all patients demonstrated improvements that continued with greater surgeon experience, with over 1,600 cases required to get a PSM rate <10%. When only pT3 patients were evaluated, the learning curve started to plateau after 1,000-1,500 cases. Mean OT plateaued after 750 cases although with further surgical experience the OTs started to climb again.
Conclusions: The learning curve for RALP is not as short as previously thought, and a large number of cases are needed to get PSM rates and OTs to a minimum. This suggests that RALP should be performed by high volume surgeons in order to optimize patient outcomes.
Disclosure: Ashutosh Tewari, MD: Research Funding from Intuitive Surgical.
Lead Author: Neil Fleshner, MD
University Health Network
Toronto, Canada
General Session 1
Thursday, February 17, 2011
8 AM-9:45 AM ET
A new study has shown that a drug commonly used to treat men with an enlarged prostate gland - dutasteride (Avodart) - may also slow the growth of early-stage prostate cancer among men participating in "active surveillance" of their disease. Active surveillance, also called watchful waiting, involves regularly monitoring men with early-stage prostate cancer to determine if the cancer is growing and needs treatment.
"In some cases, we treat prostate cancer that may never become life-threatening. I'm hoping that these results, showing that men may be able to take a drug that slows the cancer's growth, may allow more men to pursue active surveillance for even longer periods," said lead author Neil Fleshner, MD, Head of Urology at the University Health Network in Toronto, Ontario, Canada, and the Love Chair in Prostate Cancer Prevention at the Princess Margaret Hospital in Toronto.
While prostate cancer is the second most common cancer in men in the United States, most cases are slow-growing and only a small percentage of men actually need treatment with surgery or radiation early on.
In the REDEEM (Reduction by Dutasteride of Clinical Progression Events in Expectant Management of Prostate Cancer) study, researchers tested whether the drug dutasteride could control the growth of low-risk, early-stage prostate cancer and further reduce the potential use of more aggressive therapy in men following watchful waiting.
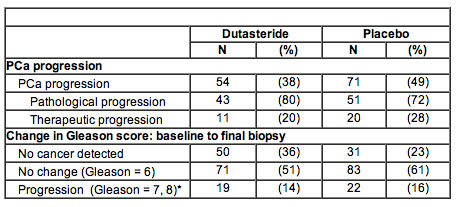
In the study, 302 men with early-stage prostate cancer were randomly given either dutasteride or placebo for three years. Prostate biopsies were taken at 18 and 36 months or if they were warranted because of indications of disease progression. Investigators found that those taking dutasteride had a longer time to cancer progression compared to those taking placebo. In the dutasteride group, 38 percent (54) of the men experienced some progression of their cancer, compared to 49 percent (71) of the placebo group. There was a reduced relative risk for cancer progression of 38.9 percent in the dutasteride group.
They also found that those men taking dutasteride had less chance of finding cancer on re-biopsy. Thirty-six percent (50) of the men in the drug group and 23 percent (31) in the placebo group had no cancer detected on their final biopsy. In addition, those who were given dutasteride had lower levels of prostate cancer-related anxiety based on results of a standardized test.
"Even though men realize that if they reach a certain age, many will have some sort of prostate cancer that likely will never give them problems, there is still anxiety associated with monitoring and not treating it," Dr. Fleshner said.
Effect of dutasteride on prostate cancer progression and cancer diagnosis on rebiopsy in the REDEEM active surveillance study.
Authors: N. Fleshner, M. S. Lucia, K. Melich, I. M. Nandy, L. Black, R. S. Rittmaster; University Health Network, Toronto, ON; University of Colorado School of Medicine, Aurora, CO; GlaxoSmithKline, Research Triangle Park, NC.
Background: The REDEEM (Reduction by Dutasteride of Clinical Progression Events in Expectant Management of Prostate Cancer) study tested whether dutasteride controlled growth of existing low risk, localized prostate cancer (PCa) and hence reduced the need for aggressive therapy in men followed with active surveillance.
Methods: 302 men, aged 48-82, with PSA <11 ng/ml, and Gleason score â≈€6 PCa (â≈€3 cores positive, <50% of any core positive) were randomized to dutasteride or placebo for 3 years. Repeat 12-core biopsies were performed at 18 and 36 months, or for-cause at other times during the study. The primary endpoint was time to progression, defined as the earliest of either pathological progression (Gleason score >6, â≈¥4 cores positive, or >50% of any core positive) or therapeutic progression (radical prostatectomy, radiation therapy, or hormonal ablation).
Results: 96% of subjects reached the primary endpoint or had a post-baseline biopsy. Dutasteride reduced time to PCa progression (relative risk reduction 38.9%, 95% CI: 12.4-57.4%, P=0.007). The table presents incidence of progression and Gleason score on final biopsy. 23% of men (N=31) in the placebo group and 36% of men (N=50) in the dutasteride group had no cancer detected on their final biopsy. PCa-related anxiety was reduced in the dutasteride arm compared to the placebo arm (P=0.036), based on the Memorial Anxiety Scale for PCa (MAX-PC). Drug-related adverse events were similar to those previously reported for dutasteride.
Conclusions: In men followed with active surveillance, dutasteride delayed the time to PCa progression, increased the percent of men with no detectable PCa, and improved PCa-related anxiety. There was no evidence of increased Gleason score upgrading with dutasteride. Dutasteride may provide a useful adjunct to active surveillance for management of PCa.
Disclosures for 2011 Genitourinary Cancers Symposium News Planning Team
Nicholas J. Vogelzang, MD: Leadership position at US Oncology, Inc.; Consultant for Amgen, Aveo, Bayer, Celgene, Dendreon, Eisai, GE Healthcare, Genentech, GlaxoSmithKline, Medscape, Novartis, Pfizer, sanofi-aventis and Wilex; Honoraria from Amgen, ArQule, Bayer, Clinical Care Options, Cougar Biotechnology, Genentech, Imedex, Lilly Lippincott, Williams and Wilkins, Medscape, Novartis, Onyx, Pfizer and Veridex; W. Robert Lee, MD: Honoraria from sanofi-aventis; Eric A. Klein, MD: Research funding from Gen Probe, Genomic Health and Abbott Labs.
American Society of Clinical Oncology
[ PRESS RELEASE ]



