ATLANTA, GA USA (UroToday) - The burden of prostate cancer in the U.S. and Europe remains significant despite the fact that overall mortality has improved. Prostate cancer remains the second leading cause of cancer death in men.
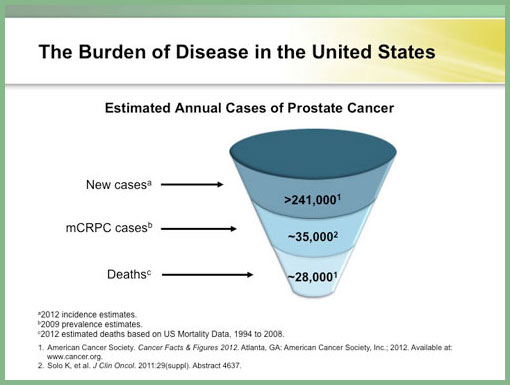
This is an exciting era for new prostate cancer treatments, and specifically metastatic castrate-resistant prostate cancer (mCRPC). The estimated number of new prostate cancer cases is 241.000 and 28,000 deaths (based on ACS 2012 Cancer Facts 2012 cancer incidence).1 Annually, there are an estimated 35,000 mCRPC cases in the U.S.2 Androgen-sensitive prostate cancer responds to therapies that decrease androgen levels.3 Androgen deprivation therapy using gonadotropin-releasing hormone (GnRH) analogs (or orchiectomy) with or without an antiandrogen is the standard approach in early disease.3, 4 The GnRH analogs affect androgen production only in the testes.3 In eugonadal men, 90% of androgens are synthesized in the testes, 10% in the adrenals.5
Although medical castration leads to a decrease in the production of testosterone, in mCRPC the prostate cells remain androgen-sensitive and widely dependent on androgens. Adrenal glands and prostate cancer tissue can produce androgens, which eventually leads to continued prostate cancer growth. Blocking androgen production by nongonadal sources is a targeted clinical benefit. One agent, abiraterone acetate (ZYTIGA®), reduces androgen production by blocking the enzyme, cytochrome P450 17 alpha-hydroxylase (CYP17). ZYTIGA® is indicated for use in combination with prednisone for the treatment of mCRPC who have received prior chemotherapy containing docetaxel. The mechanism of action is abiraterone which inhibits the production of androgen in three sites: the testes; the adrenal glands and impacts the prostate tumor tissue directly.

Abiraterone acetate, taken in oral form, is converted in vitro to abiraterone.3 The patients in the abiraterone acetate clinical trial were using GnRH agonist or were previously treated with orchiectomy and were required to have a serum testosterone level ≥ 50 ng/dL3. This drug works at CYP17: 17α-hydroxylase and CYP17: C17, 20-lyase level.

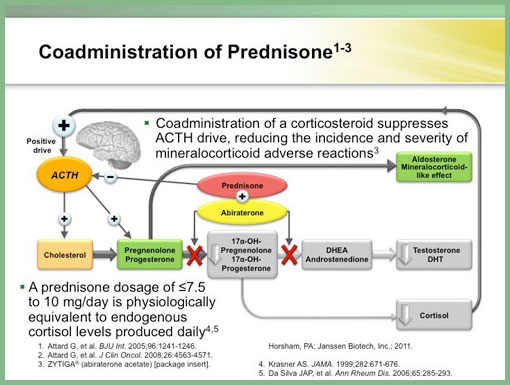
Treatment with ZYTIGA® results in the inhibition of androgen production. The result is decreased serum testosterone and other androgens in patients in the placebo-controlled phase III clinical trial. Low-dose prednisone is prescribed and it is not necessary to monitor the effects of abiraterone acetate on the serum testosterone levels. With this drug there is a potential mineralocorticoid effect. During the CYP17 inhibition, the cortisol production is blocked resulting in decreased cortisol levels. The reduced cortisol levels generate a positive ACTH pathway to stimulate cortisol production. Since the cortisol production is blocked, there is the potential to increase mineralocorticoid levels. For this reason potential mineralocorticoid excess should be monitored as a side effect in the form of hypertension, hypokalemia and/or fluid retention. In particular, when assessing the patient make a complete history of cardiovascular disease or other medical conditions that may be compromised by these side effects.
The recommended dosage for ZYTIGA® is 1,000 mg (four 250-mg tablets) taken orally on an empty stomach in combination with prednisone (5 mg, administered twice daily). The administration of prednisone suppresses the ACTH drive, reducing the incidence and severity of mineralocorticoid adverse reactions.
Overview: Results of the Phase III Abiraterone Clinical Trial
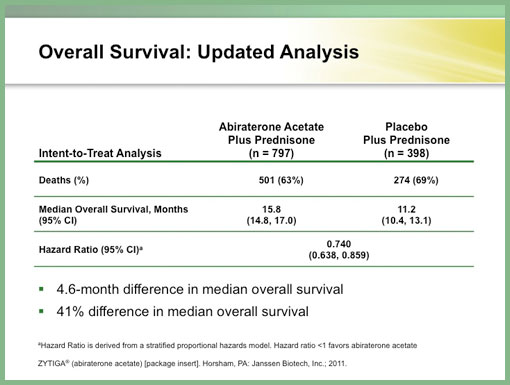
The Phase III trial began in April 2008 with 1,195 patients randomized with docetaxel-refractory CRPC to either abiraterone or placebo in a 2:1 fashion. Both arms received prednisone 5 mg twice daily. The independent data monitoring committee recommended the study be unblinded on August 20, 2010. By this point, abiraterone-treated patients had received a median of eight 28-day cycles, and the patients in the placebo arm had received a median four 28-day cycles. The study demonstrated a median overall survival in the abiraterone-treated arm of 14.8 months compared with 10.9 months in the placebo arm. The abiraterone arm also yielded superior outcomes in time to PSA progression (10.2 months versus 6.6 months, P < 0.0001), radiographic progression-free survival (5.6 months versus 3.6 months P < 0.0001), and PSA declines ≥50% (confirmed, 29.1% versus 5.5%, P < 0.0001). The U.S. Food and Drug Administration approved ZYTIGA® on April 28, 2011. The recommended dose is 1000 mg daily along with prednisone 5 mg twice daily. The most common adverse effects seen on abiraterone therapy were joint discomfort, hypertension, and hypokalemia.
Take-away messages from Dr. Moul:
“This is important for us as urologist to recognize that the landscape for prostate cancer is changing. That includes our role in all aspects of prostate cancer treatment from early to end-stage disease. Looking at what is approved and those drugs coming down the pike, ZYTIGA®® (abiraterone acetate) is a hormonal agent currently approved by the FDA for the treatment of metastatic castration-resistant prostate cancer after a patient has progressed on docetaxel. ZYTIGA® is also being investigated for its usefulness prior to chemotherapy and those results look very promising.
ZYTIGA® has many key clinical points when prescribing this drug. In its oral form, ZYTIGA® does have some side effects, but in combination with low-dose prednisone the mineralocortoid excess is reduced. A key teaching point is to know how abiraterone acetate works. It works in three separate sites: the testes, the adrenal glands and the prostate tumor tissue. Most importantly, abiraterone blocks at the CYP17: 17α-hydroxylase and CYP17: C17, 20-lyase level.
The way the drug works; it can increase the pathway to mineralocorticoid. If you use ZYTIGA® without a steroid, it could cause adverse events such as hypertension, hypokalemia or fluid retention. For this reason, the drug is taken with low dose prednisone given twice a day and by doing that the prednisone helps block adrenocortical insufficiency. When first starting ZYTIGA®, the FDA requires that the patient’s liver function be monitored every two weeks.
A key patient instruction point is taking this oral agent in the right way, in terms of diet and what’s in their stomach. I recommend the patient take ZYTIGA® before breakfast. The ideal time is 2 hours before breakfast, then waiting one hour before staring breakfast and then taking prednisone with breakfast and with dinner.
In May of 2012 ZYTIGA® was officially approved for the treatment of (mCRPC) in men who have progressed on docetaxel.
In summary, ZYTIGA® (abiraterone acetate) inhibits CYP17, an enzyme complex needed for androgen biosynthesis, and when used in combination with prednisone, improved overall survival in patients with mCRPC who received prior chemotherapy containing docetaxel. The clinical trial demonstrated a survival advantage of 4.6 month, that’s a 41% difference in median overall survival which was highly statistically significant. Overall, ZYTIGA® has a good safety profile.”
When asked when to stop ZYTIGA®, Dr. Moul replied, “As we found in the study, the patient is discontinued on this treatment plan if all three criteria for disease progression are met: 1) a 25% increase in PSA over baseline; 2) protocol-defined radiographic progression; or 3) symptomatic or clinical progression.” Dr. Moul reminded colleagues during the session that ZYTIGA® should not be stopped simply due to a rise in PSA.
References:
- American Cancer Society. Cancer Facts & Figures 2012. Atlanta, GA; American Cancer Society, Inc.: 2012. Available at: www.cancer.org.
- Solo K, et al. J Clin Oncol. 2011: 29 (suppl). Abstract 4637.
- ZYTIGA®® (abiraterone acetate) [package insert]. Horsham, PA; Janssen Biotech, Inc.: 2011.
- National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology. Version 4.2011. Available at www.nccn.org.
- Holmes CJ, et al. Ther Adv Med Oncol. 2010: 2:107-123.
Additional reading:
- Johann S. de Bono, et al. (for the COU-AA-301 Investigators). Abiraterone and Increased Survival in Metastatic Prostate Cancer. N Engl J Med. 2011; 364:1995-2005.
- Rehman Y. Rosenberg J. Abiraterone acetate: oral androgen biosynthesis inhibitor for treatment of castration-resistant prostate cancer. Drug Des Devel Ther. 2012; 6: 13–18.
Presented by Judd Moul, MD* at the American Urological Association (AUA) Annual Meeting - May 19 - 23, 2012 - Georgia World Congress Center - Atlanta, GA U.S.A
*James H. Semans, MD, Professor of Surgery
Director, Duke Prostate Center

Keywords: abiraterone acetate, CYP17, inhibitors, androgens, metastatic castration-resistant prostate cancer (mCRPC), prostate cancer




