BERKELEY, CA (UroToday.com) -
Tumor-shed vesicles mediate tumor–stroma interactions in acid ceramidase over expression cells
It has been shown in our published data that acid ceramidase (AC) is up-regulated at the protein level in a majority of prostate cancer tissues compared to their adjacent normal controls.[1] Recent studies suggested that increased expression of AC causes secretion of the matrix-degrading protease cathepsin B (CatB). While elucidating regulation of CatB by AC, we observed, that microvesicle shedding might be a secretory pathway for CatB release from cells that over express AC. Scanning electron microscopic analysis revealed enhanced shedding of vesicles 100nm-300nm in diameter in PPC1 human prostate cancer cells transfected with AC.
These data lead us to examine whether vesicles prepared from AC over-expressing cells contained CatB. Western blot analysis demonstrated the presence of CatB in vesicles shed from prostate cancer cell lines PPC1 and DU145, with a substantial increase in CatB level in vesicles from AC overexpressing cells. This suggests that increased CatB secretion may be through increased vesicles generation induced by AC. AC and CatB are both localized in lysosomes with acidic pH optima. To determine whether pH plays a role in activation of AC and CatB in vesicles, pelleted vesicles were re-suspended in PBS at acidic and neutral pH. Figure 2 shows that no significant difference in active bands of CatB was observed at either pH, suggesting that pH-dependent activation of CatB does not occur. This is consistent with a previous report that CatB undergoes autoproteolytic cleavage, also at neutral pH.[2] However, a significant increase of the active band of AC at the lower pH was detected in vesicles from both prostate cancer cell lines. This finding reveals that acidic pH may impact the activation of AC in tumor-shed vesicles, which may, in turn, promote its anti-apoptotic function under conditions of acidic pH, as frequently found in the tumor microenvironment. When we examine the sphingolipid levels in vesicles isolated from AC over-expressing cells, we observed a lower level of total ceramide in both pH conditions relative to GFP control (Figure 3A). AC is known to catalyze the hydrolysis of ceramide into free fatty acid and sphingosine, and our data demonstrated increased levels of sphingosine consistent with this and corresponding to the production of active AC as shown in Figure 2, again confirming the pH-dependent activation of AC in vesicles.
Vesicles shed by cancer cells are known to mediate tumor-host interactions. Therefore, we hypothesize that AC over expression promotes release of tumor-shed vesicles that may contain important components, including CatB, that are able to play a role in modification of the tumor microenvironment. Further studies will address the impact of these microvesicles on tumor–stromal interaction and the mechanism for microvesicle formation and release.
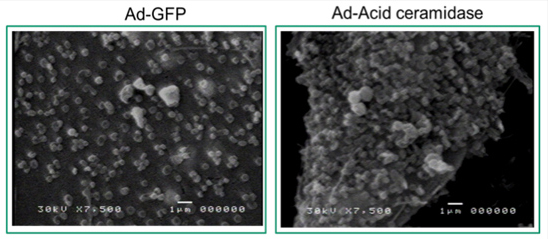
Figure 1: Scanning electron microscopy of PPC-1 cell vesicle shedding (30 hours post infection). Left: PPC-1 cells infected with Ad-GFP. Right: PPC1 cells infected with Ad- Acid ceramidase.
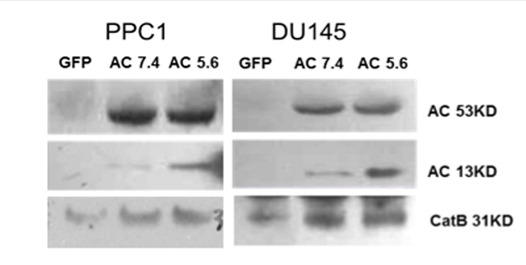
Figure 2: Presence of CatB in AC overexpressing cell-shed vesicles. PPC1 cells were infected by either Ad-AC or Ad-GFP. 24 hours after infection, cells were cultured for addition 16 hours in serum free medium. Vesicles were prepared as previous described [1]. Pelleted microvesicles were re-suspended in PBS at two different pH values (5.6 and 7.4). The presence of CatB expression and AC expression in tumor-shed microvesicles was examined by western blotting.

Figure 3: PPC1 cells were infected by either Ad-AC or Ad-GFP. 24 hours after infection, cells were cultured for addition 16 hours in serum free medium. Vesicles were prepared as previous described.[1] Pelleted vesicles were re-suspended in PBS at two different pH values (5.6 and 7.4). The level of sphingolipids in tumor-shed vesicles was analyzed by mass spectrometry.
References:
- Norris, J.S., et al., Combined therapeutic use of AdGFPFasL and small molecule inhibitors of ceramide metabolism in prostate and head and neck cancers: a status report. Cancer Gene Ther, 2006. 13(12): p. 1045-51.
- Buck, M.R., et al., Degradation of extracellular-matrix proteins by human cathepsin B from normal and tumour tissues. Biochem J, 1992. 282 ( Pt 1): p. 273-8.
Written by:
Thomas Beckham and Xiang Liu as part of Beyond the Abstract on UroToday.com. This initiative offers a method of publishing for the professional urology community. Authors are given an opportunity to expand on the circumstances, limitations etc... of their research by referencing the published abstract.
Department of Microbiology and Immunology, Medical University of South Carolina, Charleston, SC USA

More Information about Beyond the Abstract


