BERKELEY, CA (UroToday.com) - PROSTVAC is an active immunotherapy composed of a series of poxviral-based vectors that express prostate-specific antigen (PSA). PROSTVAC immunotherapy is based on a prime-boost regimen; the first PROSTVAC administration uses vaccinia vector to prime the immune system, and the second and subsequent boost doses are fowlpox-based. This regimen is designed to optimize the immune response by exposing the immune system to the same antigens while using different vectors. The vectors also express a triad of human T cell costimulatory molecules, B7.1, ICAM-1, and LFA-2, which are known as TRICOM.
PROSTVAC as an active immunotherapy candidate specifically targets prostate cancer cells by presenting PSA plus the 3 costimulatory molecules to the immune system. Preclinical data suggest that the immune system responds with PSA-specific T cells that attack and kill tumor cells. As the prostate cancer cells die, they release other proteins or protein fragments that allow the immune system to spread its attack to these other cancer-associated proteins. This process is called antigen spreading or antigen cascade and generates a progressively expanding and long-term immune response (Figure 1). The accompanying video provides a more detailed description of the mechanism of action.
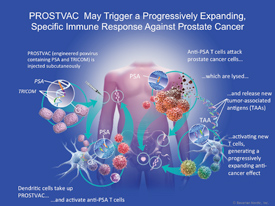 |
| Figure 1. PROSTVAC sesigned to trigger a progressively expanding, specific immune response against prostate cancer |
A multicenter, randomized, phase 2 study of PROSTVAC in patients with metastatic castration-resistant prostate cancer (CRPC) showed evidence of a significantly improved overall survival, with an estimated median survival of 25.1 months for PROSTVAC compared with 16.6 months for placebo (log-rank P=0.006) (Kantoff et al. 2010). Based on these results, an international, randomized, placebo-controlled phase 3 study is currently being conducted in 1 200 patients with asymptomatic or minimally symptomatic metastatic CRPC (PROSPECT, NCT01322490).
In a study published in Cancer Immunology Research (2014;2:133-141), raw data from multiple clinical studies of PROSTVAC were analyzed to gain additional understanding about the effects of this immunotherapy on tumor-specific T cells. After PROSTVAC administration, 57% (59/104) of patients had a ≥ 2-fold increase in PSA-specific T cells (median 5-fold increase). Furthermore, the median number of PSA-specific T cells was 30 per million peripheral blood mononuclear cells (PBMCs), which was comparable to the median baseline level of influenza-specific T cells (33.3 per million PBMCs), implying potent immunogenicity and a clinically meaningful T cell response. Importantly, these T cells were measured in peripheral blood. Preclinical evidence has shown that tumor-specific T cells are enriched at the tumor site, suggesting that levels of PSA-specific T cells may be even higher in the tumor.
In the same study, other aspects of the immune response were evaluated. Data from 28 patients were tested for antigen spreading. Of these 28 patients, 19 (68%) patients showed evidence of antigen spreading after PROSTVAC subcutaneous administration, with 9 patients having a positive response to 2 or more antigens other than PSA. Also, regulatory T cells were decreased in number and suppressive functionality following PROSTVAC administration, which was associated with a trend toward improved overall survival. Lastly, an analysis of antibody responses in 349 patients who received PROSTVAC in several studies found evidence of PSA antibody response in just 2 patients. These findings suggest that PROSTVAC may activate a T cell predominant immune response.
Additional data on the mechanism of action of PROSTVAC from preclinical studies were presented at the 2014 annual meeting of the American Society for Clinical Oncology and are provided as an extension of the clinical data (Mandl et al. 2014). These preclinical data suggest that immunotherapy with PROSTVAC initiates a robust and prolonged immune response against prostate cancer cells and may break immunologic tolerance to PSA. When administered in the prime-boost regimen, PROSTVAC enhanced the magnitude and quality of PSA-activated CD4 and CD8 T cell responses. These responses were characterized by highly functional effector T cells that produced multiple cytokines with cytotoxic activity. The anti-tumor efficacy of PROSTVAC in a transplantable prostate cancer model was dependent on both CD4 and CD8 T cell immune responses, and antigen spreading of immune response to endogenous tumor antigens was observed. PROSTVAC also greatly increased the ratio of activated effector T cells to regulatory T cells in the tumor.
These data suggest that PROSTVAC elicits T cell-mediated immune responses to PSA and other tumor-associated antigens. Ongoing preclinical and clinical studies will further elucidate the mechanism of action and determine the role of PROSTVAC in the treatment of prostate cancer patients.
References:
Kantoff PW, Schuetz TJ, Blumenstein BA, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099-1105.
Mandl SJ, Rountree RB, Owen RB, dela Cruz T, Hwang O, Paranjpe G, Enstrom A, Franzusoff A, Goessl C, Breitmeyer JB. PROSTVAC, PSA-targeted immunotherapy: new evidence for mechanism of action. J Clin Oncol. 2014;32:5s (suppl; abstr 3080).
Shore ND. PROSTVAC targeted immunotherapy candidate for prostate cancer. Immunotherapy. 2014;6(3):235-247.
Written by:
James L. Gulley, MD, PhD as part of Beyond the Abstract on UroToday.com. This initiative offers a method of publishing for the professional urology community. Authors are given an opportunity to expand on the circumstances, limitations etc... of their research by referencing the published abstract.
Laboratory of Tumor Immunology and Biology, Center for Cancer Research, National Cancer Institute, NIH, 10 Center Drive, 13N208, MSC-1750, Bethesda, MD 20892 USA
Immune impact induced by PROSTVAC (PSA-TRICOM), a therapeutic vaccine for prostate cancer - Abstract
More Information about Beyond the Abstract


