We aimed to assess the outcome of patients treated with second-line cabozantinib for mRCC primary refractory to first-line therapy defined as Response Evaluation Criteria in Solid Tumors (RECIST) progression in the computed tomography scan as the best response to the upfront treatment.
We retrospectively collected data from 11 worldwide centers. Overall survival (OS) and progression free survival (PFS) were analyzed using Kaplan-Meier curves. Cox proportional models were used in univariate and multivariate analyses.
We collected data from 108 patients with mRCC primary refractory to pembrolizumab plus axitinib (17%), nivolumab plus ipilimumab (36%), or tyrosine kinase inhibitors (TKIs; 31% sunitinib and 16% pazopanib). The median OS with cabozantinib was 9.11 mo, and it was 8.84 and 9.11 mo in patients primary refractory to immunocombinations and TKIs, respectively (p = 0.952). A significant difference was found between patients' primary refractory to pembrolizumab plus axitinib (OS not reached) and those primary refractory to nivolumab plus ipilimumab (median OS 8.12 mo, p = 0.024). The median PFS with cabozantinib was 7.30 mo, without significant differences between patients primary refractory to immunocombinations and those primary refractory to TKIs (6.90 vs 7.59 mo, p = 0.435) or between patients primary refractory to pembrolizumab plus axitinib and those primary refractory to nivolumab plus ipilimumab (7.92 and 6.02, p = 0.509). Investigator assessed overall response rates were 21% and 12% in patients primary refractory to first-line immunocombinations and TKIs, respectively, with a clinical benefit of 48% in the overall population.
Our data show that cabozantinib is active in primary refractory mRCC patients regardless of which treatment is received as first-line therapy. Systemic options and prognosis of primary refractory patients with mRCC, particularly those treated with novel immune-based combos, are among the major challenges that we need to face in this field.
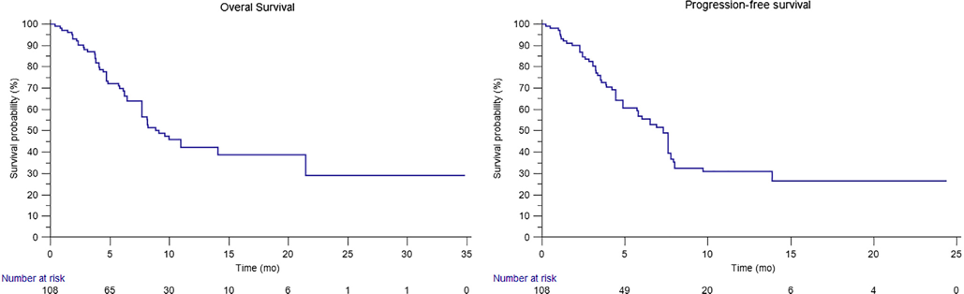
Overall survival (OS) and progression-free survival (PFS) of mRCC patients primary refractory to first-line therapy treated with second-line cabozantinib. The median OS and median PFS from the start of cabozantinib were 9.11 (95% CI 7.69–21.44) and 7.30 (95% CI 4.87–7.59) mo, respectively. CI = confidence interval; mRCC = metastatic renal cell carcinoma.

Overall survival (OS) and progression-free survival (PFS) of cabozantinib as second-line therapy in mRCC patients primary refractory to first-line immunocombinations or TKIs. The median OS was 8.84 mo in patients primary refractory to first-line immunocombinations (95% CI 6.44–21.44) and 9.11 mo (95% CI 7.69–10.95) in patients primary refractory to first-line TKIs (p = 0.952); as regards PFS, no significant difference (p = 0.435) was found between the two groups — 6.90 (95% CI 4.87–13.87) and 7.59 (95% CI 4.44–7.76) mo, respectively. CI = confidence interval; mRCC = metastatic renal cell carcinoma; TKI = tyrosine kinase inhibitor
Written by: Matteo Santoni1 and Francesco Massari2
- Oncology Unit, Macerata Hospital, Macerata, Italy
- Medical Oncology, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italia
Read the Abstract


