While miRNAs have been proposed as biomarkers in a variety of diseases and tumor entities, in their majority there has not been any further characterisation as to their intracellular effects. In contrast, from miRNA15a we know though research from others and from us1 that it is induced mainly via the A type receptor of Endothelin-1, being a well-known activator in renal cell carcinoma.2,3
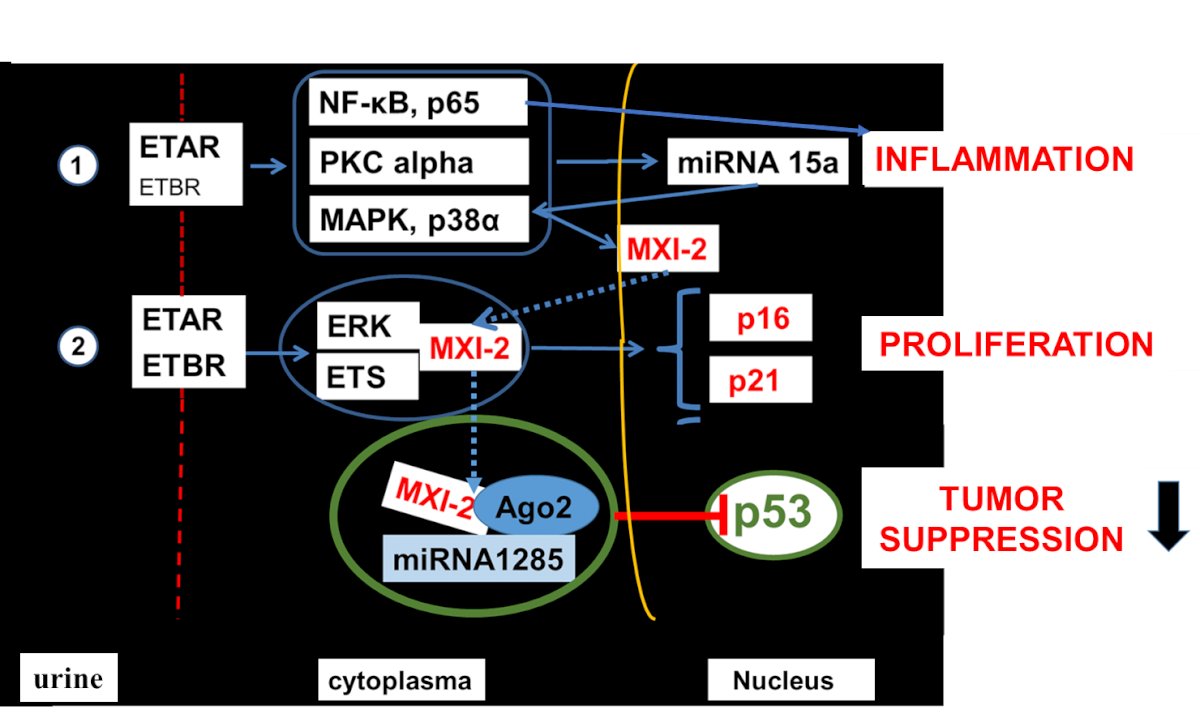
This activation prevents a complex formation consisting of protein kinase C (PKC) alpha with mitogen-activated protein kinase (MAPK) p38 alpha. This complex would be able to migrate from the cytoplasm into the nucleus, due to the nuclear location sequence of MAPK to gain nuclear access.4 This lack of migration has two effects: i) the control of the stability of intranuclear levels of pri-mRNA15a is lost; ii) the third partner of the cytoplasmic complex, nuclear factor kappa B (NF-kB) is unrestrictedly able to transmigrate into the nucleus, upregulating inflammatory genes having a NF-kB binding site in their promoter (i.e. VCAM-1,5 IL-6,6 fractalkine,7 as well as ET-1,8). Nuclear PKC alpha levels become low, pri-miRNA15a is activated, released into the cytoplasm to become a mature miRNA. As a novel mechanism this miRNA is remigrating into the nucleus, where it leads to a DNA truncation of MAPK,9 resulting in a protein known as Mxi-2 in the literature. Mxi-2 has several effects in the cytoplasm: it serves as transcription factor together with ETS-1 and ERK to induce proliferation after translocating into the nucleus, where the complex binds via ETS1 to the ETS-binding site in the p16INK4a promoter, initiating p16INK4a gene expression.10
Furthermore, it leads to p53 downregulation in a complex together with Ago2 and miRNA1285,11 counteracting tumor growth [unpublished]. Subsequently, in the urines of renal carcinoma one can find upregulated miRNA15a levels and low PKC alpha and Mxi-2 levels.12,13 Low levels of p16INK4a14 and p5315 have been described in ccRCCs and are associated with an improved diagnosis.
Written by:
- Sanjay Mathur, FACerS, FASM, FEurASc, FNA, Institute of Inorganic Chemistry, University of Cologne, Cologne, Germany
- Jochen W. U. Fries, MD, Institute of Pathology, University of Cologne, Cologne, Germany
References:
- Fries JWU. MicroRNAs as markers to monitor Endothelin-1 signaling and potential treatment in renal disease: proteinuric damage – carcinoma - toxicity. Biol Cell. (2019). doi: 10.1111/boc.201800059. Review.
- Pflug BR, Zheng H, Udan MS, D'Antonio JM , Marshall FF, Brooks JD, Nelson JB. Endothelin-1 promotes cell survival in renal cell carcinoma through the ETA receptor. Cancer Letters 246: 139-148 (2007).
- von Brandenstein M, Depping R, Schäfer E, DienesH.-P, Fries JWU. Protein kinase Cα regulates nuclear pri-microRNA 15a release as part of endothelin signalling. BBA 1813: 1793-1802 (2011).
- Gerstung M, Roth T, Dienes H-P, Licht Chr, Fries JWU. Endothelin-1 induces NF-kB via two independent pathways in human renal tubular epithelial cells. Am J Nephrol 27: 294-300 (2007).
- Seron D, Cameron JS, Haskard DO. Expression of VCAM-1 in the normal and diseased kidney. Nephrol. Dial. Transpl. 6: 917-922 (1991).
- Boswell RN, Yard BA, Schramma E, van Es LA, Daha MR, van der Woude FJ. Interleukin-6 production by human proximal tubular epithelial cells in vitro: analysis of the effects of interleukin-1ά (IL-1ά) and other cytokines. Nephrol. Dial. Transpl. 9: 599 – 606 (1994).
- Donadelli R, Zanchi C, Morigi M, Buelli S, Batani C, Tomasoni S, Corna D, Rottoli D, . Benigni A, Abbate M, Remuzzi G, Zoja C. Protein overload induces fractalkine upregulation in proximal tubular cells through nuclear factor kappa B- and p38 mitogen-activated protein kinase pathways. J. Am. Soc. Nephrol. 14: 2436 – 2446 (2003).
- von Brandenstein M, Pandarakalam JJ, Kroon L, Loeser H, Herden J, Braun G, Wendland K, Dienes H.-P., Engelmann U, Fries JWU. MicroRNA15a, inversely correlated to PKCα, is a potential marker to differentiate between benign versus malignant renal tumors in biopsies and in urine samples. Am J Pathol 180: 1787-1797, (2012a).
- von Brandenstein M, Bernhart SH, Pansky A, Richter C, Kohl T, Deckert M, Heidenreich A, Stadler PF, Montesinos-Rongen M, Fries JWU. Beyond the 3’UTR binding – microRNA - induced protein truncation via DNA binding. Oncotarget 9: 32855-32867 (2018).
- von Brandenstein M, Schlosser M, Richter C, Depping R, Fries JW. ETS-dependent p16INK4a and p21waf1/cip1 gene expression upon endothelin-1 stimulation in malignant versus and non-malignant proximal tubule cells. Life Sci. 91:562-71 (2012b).
- Köditz B, Fries JWU, Göbel H, Paffenholz P, Richter K, Heidenreich A, von Brandenstein M. Mxi-2 Dependent Regulation of p53 in Prostate Cancer. Anticancer Res. 40:5539-5544. doi: 10.21873/anticanres.14566 (2020).
- Von Brandenstein M, Köditz B, Eich C, Cahir S, Heidenreich A, Fries JWU. NON-invasive urine markers for the differentiation between RCCs and Oncocytoma. J Clin Lab Anal. 2021 35: e23762. doi: 10.1002
- Köditz M, von Brandenstein M, Huerta-Arana M, Heidenreich A, Fries JWU. Novel noninvasive marker of regression of clear cell renal cell carcinoma (ccRCC). Turk J Urol 2022; 48: 49-57; doi: 10.5152/tud.2022.21259
- Latic D, Radojevic-Skodric S, Nikolic S, Prvanovic M, Lazic M, Dzamic Z, Bogdanovic L, Radunovic M, Vukovic M. Immunohistochemical study of cyclin A and p16 expression in patients with renal cell carcinoma. J BUON. 22:1322-1327 (2017).
- Gurova KV, Hill JE, Razorenova OV, Chumakov PM, Gudkov AV. p53 pathway in renal cell carcinoma is repressed by a dominant mechanism. Cancer Res.64:1951-1958. (2004). doi: 10.1158/0008-5472.can-03-1541.
Read the Abstract


