However, radiological staging provides minimal prognostic information for the large patient subgroup with disease control, especially for patients treated with ICIs.1 Real-time determination of benefits in patients with disease control is critical for informing clinical decisions, guiding effective therapy continuation, and avoiding unnecessary toxicity or costs. In this context, there is a need for complementary markers that can assess therapy response and outcomes in conjunction with imaging.
The modified Glasgow prognostic score (mGPS) is a simple score based on the two serum markers C-reactive protein (CRP) and albumin, which predicts outcomes more accurately than the IMDC score for patients with metastatic renal cell carcinoma (mRCC).2
The presented study aimed to investigate the prognostic and predictive value of longitudinal/ on-treatment mGPS in patients with mRCC treated with atezolizumab (+bevacizumab) or sunitinib in two independent clinical trials: the IMmotion151 study (discovery cohort) and IMmotion150 (validation cohort).3 We demonstrated that on-treatment mGPS provides valuable prognostic information, especially in the patient subset with disease control (>80%), where standard imaging has limited discriminatory power (Figure 1). The findings are validated in two independent clinical trials, supporting the integration of on-treatment mGPS into routine clinical practice for improved patient monitoring.
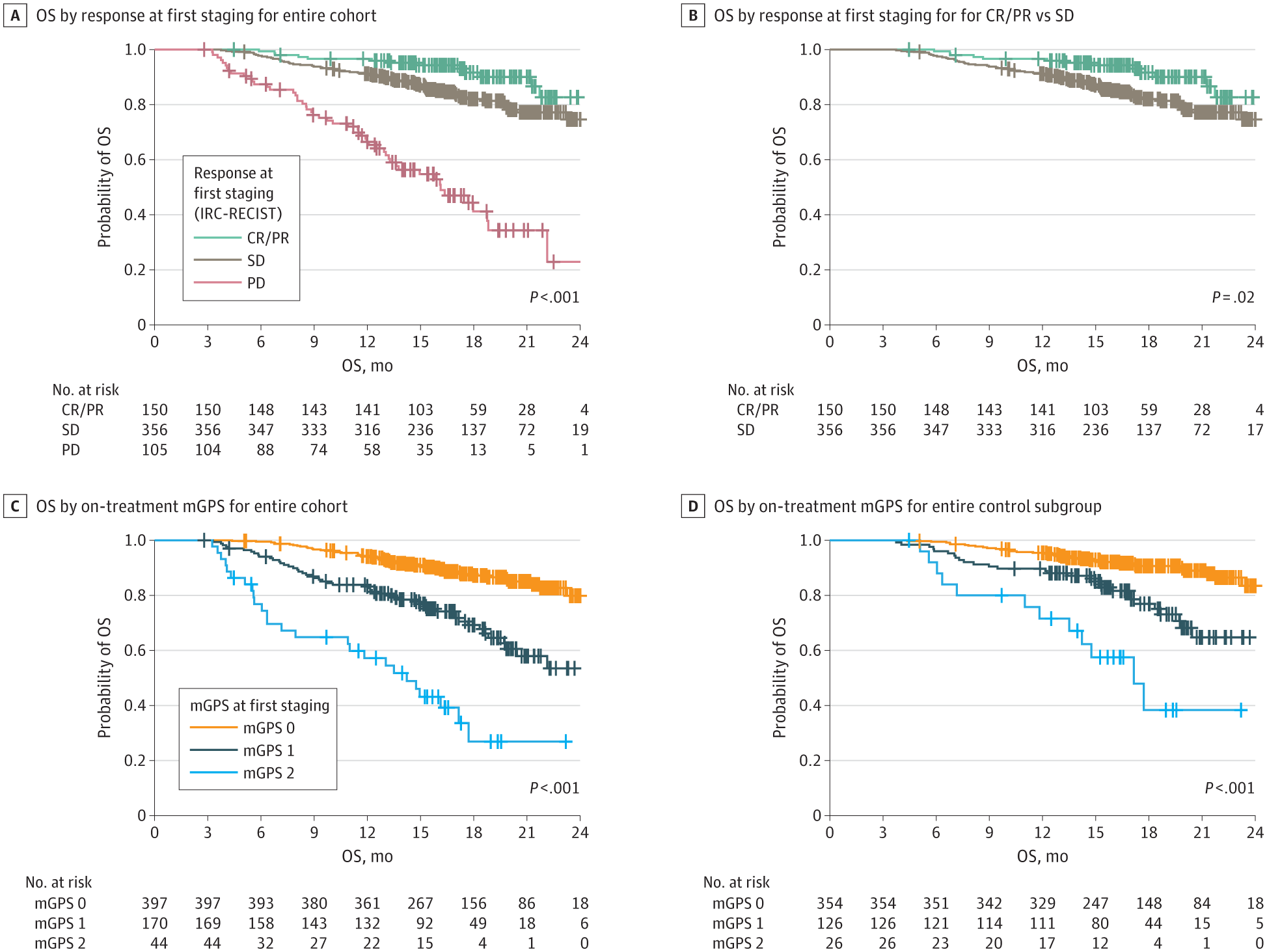
Figure 1: Prognostic Information of the On-Treatment Modified Glasgow Prognostic Score (mGPS) in the Disease Control Subgroup of the Phase 3 IMmotion151 Trial
Progressive disease (PD) in the first staging according to the Response Evaluation Criteria in Solid Tumors assessed by the Independent Review Committee (IRC-RECIST) is associated with unfavorable overall survival (OS), whereas outcomes in the complete or partial remission (CR/PR) versus stable disease (SD) subgroups differs only slightly. The on-treatment mGPS at first staging has a strong prognostic value, even in the disease control subgroup (ie, patients with CR/PR or SD), and identifies a high-risk group of patients.
The mGPS is a simple score that can be measured cost-effectively in any clinical laboratory, making it easily implementable in everyday clinical practice, even in low-resource settings. We, therefore, suggest including baseline and on-treatment mGPS measurements for clinical care and in future clinical trial protocols to further establish the role of mGPS in improving prognostication and therapy monitoring for patients with mRCC. As the mGPS is not only a measure of tumor dynamics but also reflects the patient's inflammatory and metabolic status, we believe longitudinal assessment of mGPS provides a more comprehensive picture of the patient's general condition than radiological assessment alone, akin a blood-based ECOG performance status.
The findings of our manuscript have been further discussed in a brilliant editorial authored by Gebreal et al, which provides further insights and interpretations regarding the data, adding depth and context to our research findings.4
While the study has strengths, including the investigation of two independent clinical trials and a large patient cohort, there are limitations. CRP measurements were taken at different time points, and confounding factors like infection or treatment-related inflammation need careful investigation in future studies. Additionally, as highlighted by Gebrael et al, the value of on-treatment mGPS in currently used first-line therapy combinations (anti-PD-1 + anti-CTLA4 or ICI + antiangiogenic TKI) remains to be determined through large randomized trials.4
In conclusion, integrating on-treatment mGPS into routine clinical practice can enhance therapy monitoring and provide a more holistic and patient-centered approach alongside radiological staging for improved response prediction in patients with mRCC. The simplicity and cost-effectiveness of mGPS make it a promising tool for risk stratification, response prediction, and outcome assessment.
Written by: Niklas Klümper, MD, Institute of Experimental Oncology, University Medical Center Bonn (UKB); Center for Integrated Oncology Aachen/Bonn/Cologne/Düsseldorf (CIO-ABCD); Department of Urology and Pediatric Urology, University Medical Center Bonn (UKB), Bonn, Germany
References:
- Luo J, Wu S, Rizvi H, Zhang Q, Egger JV, Osorio JC, et al. Deciphering radiological stable disease to immune checkpoint inhibitors. Annals of Oncology [Internet]. 2022 May [cited 2022 May 23];S0923753422011401.
- Saal J, Bald T, Hölzel M, Ritter M, Brossart P, Ellinger J, et al. In the phase 3 IMmotion151 trial of metastatic renal cell carcinoma the easy-to-implement modified Glasgow prognostic score predicts outcome more accurately than the IMDC score. Ann Oncol. 2022 Jun 15;S0923-7534(22)01730-6.
- Saal J, Bald T, Eckstein M, Ralser DJ, Ritter M, Brossart P, et al. Integrating On-Treatment Modified Glasgow Prognostic Score and Imaging to Predict Response and Outcomes in Metastatic Renal Cell Carcinoma. JAMA Oncol. 2023 Jun 22
- Gebrael G, Fortuna GG, Agarwal N. Developing an Ideal Risk Stratification Model for Metastatic Renal Cell Carcinoma. JAMA Oncol. 2023 Jun 22


