While significant progress has been made, further research is needed to elucidate the mechanisms of long-term response, identify reliable predictors, and develop strategies to increase the proportion of patients who benefit from these transformative therapies.
The meet-URO15 study is a multicenter retrospective study that investigated the prognostic role of peripheral-blood inflammatory indices and clinical factors to develop a novel prognostic score in mRCC patients receiving at least second-line nivolumab.
The long-term responders sub-analysis provides a significant contribution to the body of knowledge surrounding the efficacy of nivolumab in treating advanced renal cell carcinoma (RCC) in patients who have previously undergone treatment. This commentary aims to delve deeper into the findings and broader implications of the study, providing a comprehensive evaluation of its strengths and areas for further exploration.
Findings and Their Implications
One of the key findings of the study is the identification of long-term responders to nivolumab, highlighting its potential as a durable treatment option for advanced RCC. The data indicate that a subset of patients exhibits sustained responses, suggesting that nivolumab can provide significant clinical benefits beyond the initial treatment phases. This finding aligns with the growing body of literature that supports the long-term efficacy of immune checkpoint inhibitors (ICIs) in various cancers.1,2 However, although nivolumab provided a survival benefit in pretreated advanced RCC patients,1 usually only a small proportion of them achieve a long-term benefit.3
The study's results underscore the importance of identifying biomarkers that can predict long-term response. The identification of such biomarkers could revolutionize the management of advanced RCC, allowing for more personalized treatment approaches. In the present analysis of Meet-URO 15, we attempted to define the clinical characteristics that correlate with longer response to nivolumab. At multivariable analysis, we found out that patients with progression-free survival (PFS) > 24 months were more likely to have a Karnofsky Performance Status ≥ 80% and less likely to have bone metastases, an International Metastatic Renal Cell Carcinoma Database Consortium intermediate-poor status, and a neutrophil-to-lymphocyte ratio (NLR) ≥ 3.2. (Figure 1).
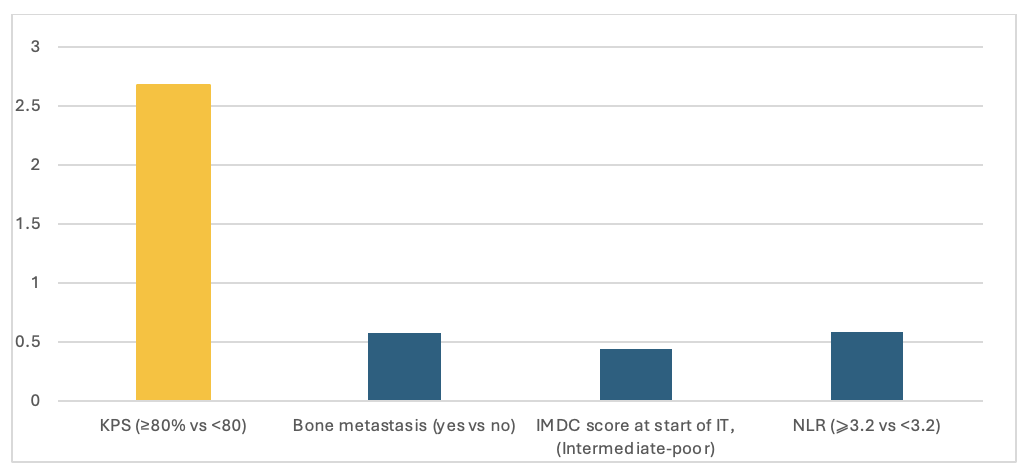
Figure 1 Multivariable analysis of the relationship of various clinical-pathological variables with PFS > 24 months.
Long-term responders exhibited a median PFS of 55.0 months versus 4.0 months of the short-term responders. The median overall survival (OS) was not reached in long-term responders while it was 17.0 months for short-term responders.
Our data are like that reported in a previous long-term response study of sunitinib and pazopanib in accordance with the general characteristics of mRCC patients.4–6 Age < 65 years, previous nephrectomy, absence of bone or lung metastases, and favorable Memorial Sloan-Kettering Cancer Center risk status were the factors associated with long-term responses in mRCC patients receiving tyrosine kinase inhibitor (TKI) as first-line therapy.4,5 Accordingly with previous studies, NLR is significantly associated with poorer OS and PFS, and lower rates of response and clinical benefit, after ICI therapy across multiple cancer types.6 While the study hints at potential biomarkers, further research is necessary to validate these findings and integrate them into clinical practice effectively.
Broader Concepts and Future Directions
The concept of long-term response to immunotherapy in oncology is gaining traction, and this study contributes valuable insights into this area. However, it also raises several questions that warrant further investigation. For instance, what are the underlying mechanisms that differentiate long-term responders from non-responders? The mechanisms are multifaceted and not yet fully understood and several hypotheses have been proposed such as immune memory tumor microenvironment modulation, and genetic and epigenetic factors.7–9 Understanding these mechanisms could pave the way for novel therapeutic strategies that enhance response rates and duration.
Additionally, the study prompts consideration of combination therapies. There is growing evidence that combining nivolumab with other agents, such as TKI or other immune checkpoint inhibitors, may enhance efficacy.10 Future studies should explore these combinations in the context of long-term responders to determine if they can further improve outcomes.
Conclusion
In summary, this study provides important insights into the durability and potential predictors of response to nivolumab in advanced RCC. While the study is methodologically sound and its findings are promising, further research is needed to fully elucidate the mechanisms of long-term response and to explore the potential of combination therapies. This commentary underscores the importance of continued investigation into the long-term efficacy of immunotherapies and the identification of predictive biomarkers, which could significantly impact the management of advanced RCC.
Long-term responders to nivolumab in previously treated advanced renal cell carcinoma: a sub-analysis of Meet-Uro 15 study
Written by: Carlo Messina,1 Martina Catalano,2 Giandomenico Roviello,2 Sara Elena Rebuzzi,3 Giuseppe Fornarini4
- Oncology Unit, A.R.N.A.S. Civico Palermo, Italy.
- Department of Health Science, University of Florence, Florence, Italy.
- Medical Oncology Unit 1, IRCCS Ospedale Policlinico San Martino of Genova, Genova, Italy
- Medical Oncology Unit, Ospedale San Paolo, Savona, Italy; Department of Internal Medicine and Medical Specialties (Di.M.I.), University of Genoa, Genoa, Italy
- Medical Oncology Unit 1, IRCCS Ospedale Policlinico San Martino of Genova, Genova, Italy
- Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med [Internet]. 2015 [cited 2023 Feb 7];373:1803–13.
- Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol [Internet]. 2014 [cited 2024 Jul 22];32:1020–30.
- McDermott DF, Choueiri TK, Puzanov I, Hodi S, Drake CG, Brahmer JR, et al. Survival, Durable Response, and Long-Term Safety in Patients With Previously Treated Advanced Renal Cell Carcinoma Receiving Nivolumab. J Clin Oncol [Internet]. 2015 [cited 2024 Feb 25];33:2013–20.
- Erman M, Biswas B, Danchaivijitr P, Chen L, Wong YF, Hashem T, et al. Correction to: Prospective observational study on Pazopanib in patients treated for advanced or metastatic renal cell carcinoma in countries in Asia Pacific, North Africa, and Middle East regions: PARACHUTE study (BMC Cancer, (2021), 21, 1, (1021), 10.118. BMC Cancer. 2021;21:1–10.
- Catalano M, De Giorgi U, Maruzzo M, Bimbatti D, Buti S, Mazzaschi G, et al. Long-Term Response to Tyrosine Kinase Inhibitors for Metastatic Renal Cell Carcinoma. Biomedicines. 2022;10:1–12.
- Valero C, Lee M, Hoen D, Weiss K, Kelly DW, Adusumilli PS, et al. Pretreatment neutrophil-to-lymphocyte ratio and mutational burden as biomarkers of tumor response to immune checkpoint inhibitors. Nat Commun [Internet]. 2021;12:1–9.
- Huang AC, Zappasodi R. A decade of checkpoint blockade immunotherapy in melanoma: understanding the molecular basis for immune sensitivity and resistance. Nat Immunol [Internet]. 2022 [cited 2024 Jul 22];23:660–70.
- Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature [Internet]. 2017 [cited 2024 Jul 22];541:321–30.
- Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science [Internet]. 2015 [cited 2024 Jul 22];348:124–8.
- Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med [Internet]. 2019 [cited 2021 Jun 3];380:1103–15.


