(UroToday.com) The 2024 American Urological Association (AUA) annual meeting featured a session on non-invasive bladder cancer, and a presentation by Dr. Hailong Hu discussing blue light cystoscopy versus white light cystoscopy for the detection of bladder cancer using modern HD 4K equipment, specifically an analysis of pivotal trial and real-world data in China.
Blue light cystoscopy using hexaminolevulinate is recommended to increase the detection of non-muscle invasive bladder cancer compared with white light cystoscopy alone. However, the benefits of Blue light cystoscopy with modern 4K LED equipment have not been assessed in a randomized controlled trial in China. This pooled meta-analysis presents data from a randomized controlled trial and a supporting real-world study conducted in China. The aim of this study was to compare hexaminolevulinate Blue light cystoscopy with white light cystoscopy in the detection of bladder cancer using state-of-the-art equipment.
In a randomized controlled trial (NCT05600322), 158 patients with known or suspected bladder cancer were enrolled in seven Chinese hospitals from November 2022 to June 2023. In the prospective real-world study, an additional 19 patients of the same inclusion criteria and measured for the same outcomes were enrolled at Hainan General Hospital from December 2022 to July 2023 and were included in the pooled analysis. Patients received intravesical hexaminolevulinate and underwent white light cystoscopy before Blue light cystoscopy. The primary endpoint was the proportion of patients with histologically confirmed tumors (Ta, T1, or CIS) who have at least one such lesion found by Blue light cystoscopy but not by white light cystoscopy. Secondary endpoints included detection of CIS, lesion-specific detection rate, false positive rates, adverse events, and satisfaction with device performance. The study design is as follows: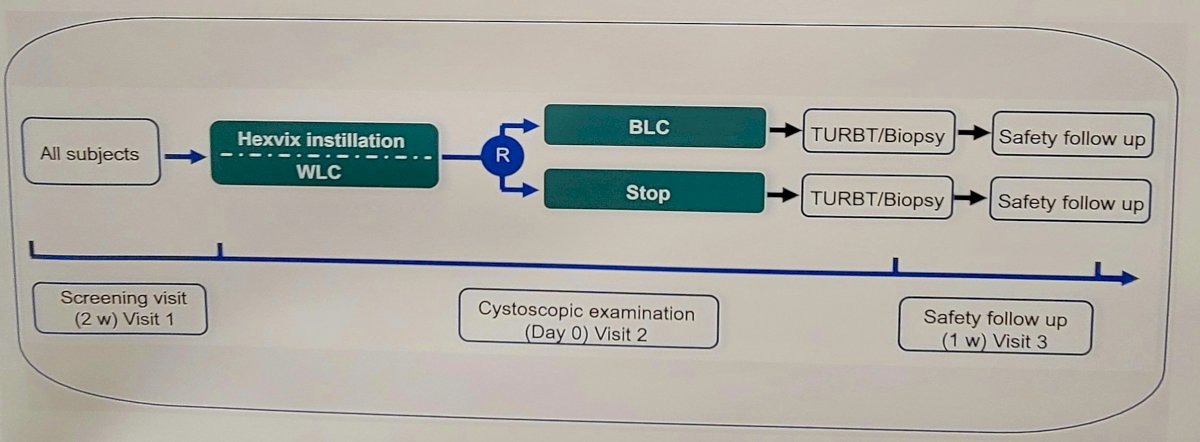
A total of 177 patients were enrolled, with 128 patients included in the full analysis set. Among 109 patients with confirmed Ta, T1, or CIS, 46 patients (42.2%), had at least one confirmed lesion found by Blue light cystoscopy but not by white light cystoscopy (p<0.0001). Among 128 patients, 11.7% (15/128) of patients had CIS, of which 80% (12/15) CIS patients showed at least 1 additional confirmed CIS lesion found by Blue light cystoscopy but not by white light cystoscopy. Detection rates for CIS was 95.2%, for Ta, T1 was 100%, and for T2-T4 tumors was 100% for Blue Light cystoscopy. Detection rates for CIS was 42.9%, for Ta, T1 was 76.5%, and for T2-T4 tumors was 100% for white light cystoscopy. The false-positive rate was 23.4% for Blue light cystoscopy and 16.0% and white light cystoscopy. The following table shows the lesion detection rate of hexaminolevulinate Blue light cystoscopy and white light cystoscopy for CIS, Ta, T2, T2-T4 and the proportion of false positive lesions detected (n = 128):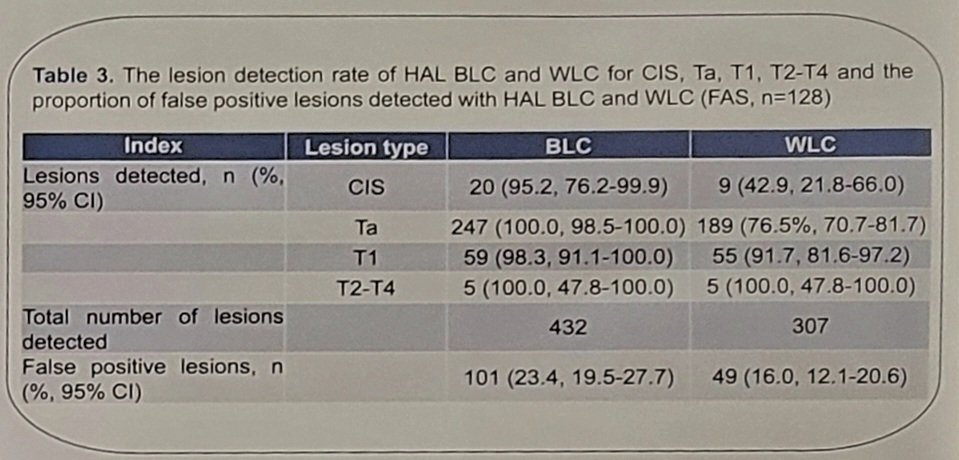
There were 209 adverse events that occurred in 110 patients, all mild to moderate, of which 200 were classified as unrelated to hexaminolevulinate. No adverse events were related to System Blue. Overall satisfaction with device performance was reported as excellent (83.8%) and good (12.4%) from the randomized controlled trial patients.
Dr. Hu concluded his presentation discussing blue light cystoscopy versus white light cystoscopy for the detection of bladder cancer using modern HD 4K equipment, specifically an analysis of pivotal trial and real-world data in China with the following take-home messages:
- Data from the first randomized controlled trial and supporting real-world study conducted in China re-confirm the superiority of hexaminolevulinate Blue light cystoscopy over white light cystoscopy, using state-of-the-art HD 4K equipment, in detecting bladder cancer, especially CIS
- Hexaminolevulinate and System Blue also demonstrate good tolerability
Presented by: Hailong Hu, MD, Professor, The Second Hospital of Tianjin Medical University, Tianjin, People’s Republic of China
Written by: Zachary Klaassen, MD, MSc - Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Urological Association (AUA) Annual Meeting, San Antonio, TX, Fri, May 3 - Mon, May 6, 2024.


