Unfortunately, progress may be moving forward too quickly, prior to having high level data to support its findings. As such, clinical trials are key to ensuring data validation. Multiple trials are ongoing utilizing PET imaging in prostate cancer.
One such study is this prospective phase II/III study utilizing 18F-DCFPyL PET/CT. 18F-DCFPyL (PyL) is a novel, high specific activity, highly selective, low-molecular weight prostate-specific membrane antigen (PSMA)-targeted PET radiopharmaceutical. In this study, they aim to assess the diagnostic accuracy of this novel imaging modality in the detection of metastatic and/or recurrent prostate cancer.
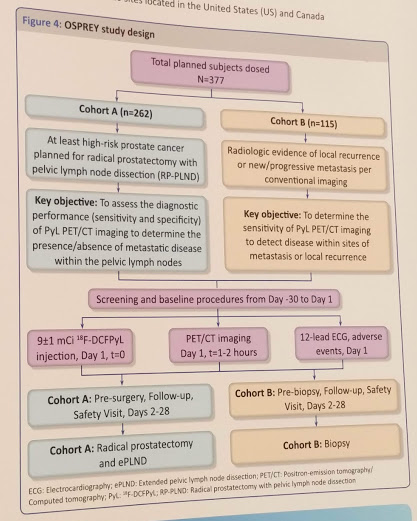
Study Design: multi-center, open-label, phase 2/3 study
Eligible Patients:
Men with at least high-risk (NCCN v3.2016) prostate cancer who are scheduled for a radical prostatectomy (Group A) or radiologically confirmed metastatic/recurrent prostate cancer (Group B).
Group A evaluates the pre-radical prostatectomy staging accuracy in the regional lymph nodes
Group B evaluates the metastatic PCa space regardless of primary therapy
All patients must be at least 18 years of age with histologically confirmed adenocarcinoma of the prostate.
Target Accrual: 400 patients
Protocol:
A single administration of PyL (9 ± 1 mCi [333 ± 37 MBq]) is administered 1-2 hours prior to PET/CT imaging on Day 1.
Objectives:
The primary objective is:
Group A: to assess the diagnostic performance (sensitivity and specificity) of PyL PET/CT in the detection of metastatic prostate cancer within the pelvic lymph nodes relative to histopathology in pre-prostatectomy patients, as determined by independent central review
Group B: diagnostic performance of PyL PET/CT in the detection of prostate cancer within sites of distant metastasis or local recurrence.
* 3 independent readers will review all PET imaging interpretation and 1 reader with reviewer conventional imaging. All 4 reviewers will blinded to all other clinical information, including pathology results.
Local pathologists remain blinded to all imaging results.
Other goals will include assessing the safety and tolerability of PyL, and pharmacokinetic parameters of PyL in a subset of subjects.
The study is already open at 10 centers and is actively accruing. As of May 2018, 354 subjects have been dosed in the study.
This trial will continue to add important data to the better understanding of the role of these novel imaging modalities to the management of prostate cancer.
Presented by: Michael J. Morris, MD
Written by: Thenappan Chandrasekar, MD, Clinical Fellow, University of Toronto, Twitter: @tchandra_uromd at the 2018 ASCO Annual Meeting - June 1-5, 2018 – Chicago, IL USA


