Cohort B included 117 men planned for image-guided biopsy of recurrent or metastatic prostate cancer who received 18F-DCFPyL, of which 93 were evaluable.
Key inclusion criteria were:
- Histologically confirmed adenocarcinoma of the prostate
- Radiographic evidence of local recurrence or new or progressive metastatic disease demonstrated on MRI/CT, ultrasound or whole-body bone scan
- Evidence of recurrence outside the confines of the prior treated site(s)
- Prior radiation or ablative therapy to the intended site of biopsy, if within the prostate bed
- Initiation of new therapy for recurrent and/or progressive metastatic disease since radiographic documentation of recurrence/progression
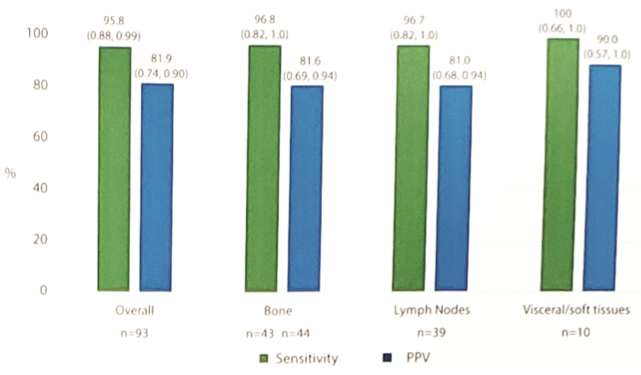
The sensitivity and positive predictive value (PPV) of 18F-DCFPyL PET/CT as compared to histopathology ranged from 92.9-98.6% (lower bound of 95% CI: 84.0-91.6%) and 81.2-87.8%, respectively. Diagnostic performance by anatomic location showed high sensitivity and high PPV in all sites of disease:
With increasing PSA, not surprisingly, PPV increased:
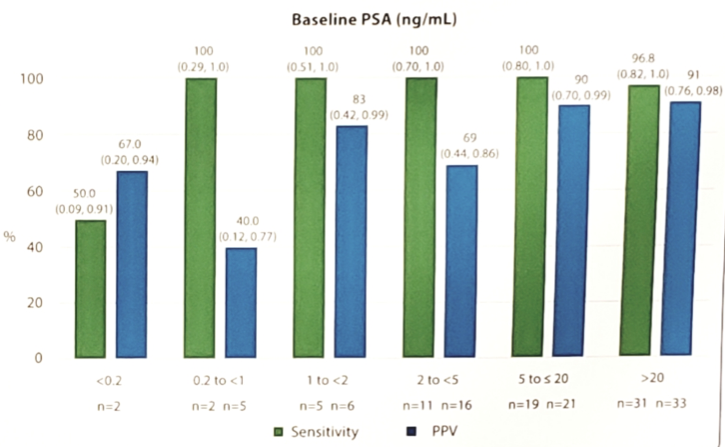
Only two cohort B patients experienced ≥1 drug-related adverse events (dysgeusia and generalized rash), and both were mild (Grade 1) in severity. The main limitation of this study is the small sample size, particularly among patients with low PSA.
Dr. Morris concluded that the results of Osprey cohort B:
- Show that 18F-DCFPyL PET/CT was well tolerated and demonstrated high sensitivity and PPV in accurately detecting nodal, bone, and visceral/soft tissue metastases
- A positive 18F-DCFPyL PET/CT scan is highly likely to represent pathologically proven distant disease, demonstrating the potential of 18F-DCFPyL as a PET imaging agent to favorably influence treatment planning
- 18F-DCFPyL is being further studies as a PET imaging agent in biochemical recurrent patients in another prospective phase 3, multi-center, multi-reader trial (CONDOR)
Presented by: Michael J. Morris, Memorial Sloan Kettering Cancer Center, New York, NY
Co-Authors: Jeremy C. Durack, Ajjai Shivaram Alva, Hebert Alberto Vargas, Morand Piert, Russell Kent Pachynski, Frederic Pouliot, Jean-Mathieu Beauregard, Mark A. Preston, Atish Dipankar Choudhury, Lawrence Saperstein, Peter Carroll, Steven P. Rowe, Kenneth J. Pienta, Tess Lin, Vivien Wong, Melissa Nichols, Jessica Donato Jensen, Barry A. Siegel; Memorial Sloan Kettering Cancer Center, New York, NY; University of Michigan, Ann Arbor, MI; Memorial Sloan-Kettering Cancer Center, New York, NY; Washington University Medical School, St. Louis, MO; CHU de Quebec and Laval University, Quebec, QC, Canada; Université Laval, Quebec City, QC, Canada; Brigham and Women's Hospital, Boston, MA; Dana-Farber Cancer Institute, Boston, MA; Yale School of Medicine, New Haven, CT; University of California San Francisco, San Francisco, CA; Department of Radiology, Johns Hopkins University School of Medicine, Baltimore, MD; James Buchanan Brady Urological Institute, Johns Hopkins University School of Medicine, Baltimore, MD; Progenics Pharmaceuticals, Inc., New York, NY; Washington University School of Medicine in St. Louis, St. Louis, MO
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md at the 2019 ASCO Annual Meeting #ASCO19, May 31- June 4, 2019, Chicago, IL USA


