San Francisco, California (UroToday.com) In this oral abstract, the author presented results from Cohort A of the OSPREY trial (NCT02981368) looking at the diagnostic performance of the PSMA-PET radiopharmaceutical 18F-DCFPyL in 252 high-risk prostate cancer patients who are to undergo radical prostatectomy with lymphadenectomy. This tracer is owned by Progenics Pharmaceuticals, Inc, and is similar to other PSMA-PET radiopharmaceuticals available such as 68Ga (Gallium-68), though the 18F radioisotope may have favorable nuclear decay properties.
PSMA is a transmembrane glycoprotein that is expressed in primary and metastatic prostate adenocarcinoma, but not neuroendocrine carcinoma. Utilizing the PSMA protein in PET/CT prostate diagnosis is of interest given the limited utility of conventional 18F-FDG PET/CT. Available data suggests that PSMA targeting PET imaging may perform better in prostate cancer relative to other PET tracers such as choline and fluciclovine. This data, however, has been limited as it is from largely retrospective analyses or single center prospective studies.
The Osprey study, therefore, was designed as a multicenter (8 US sites, 2 Canadian sites) prospective phase 2/3 trial to evaluate the diagnostic performance in 18F-DCFPyL in the initial staging of high-risk prostate cancer (Cohort A) or detection of recurrent prostate cancer (Cohort B). Three central and independent PSMA PET/CT readers were utilized, and all readers were blinded to all clinical information. The study enrollment, baseline patient characteristics, and endpoints are shown below.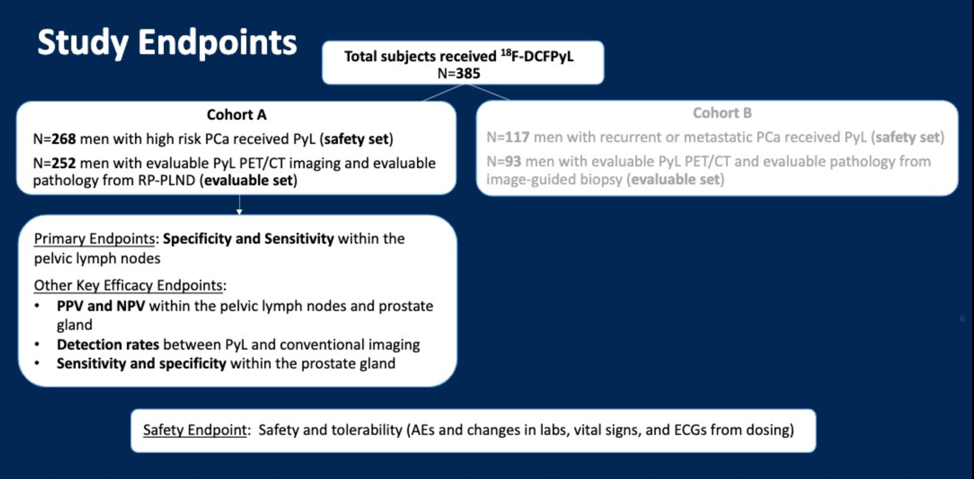
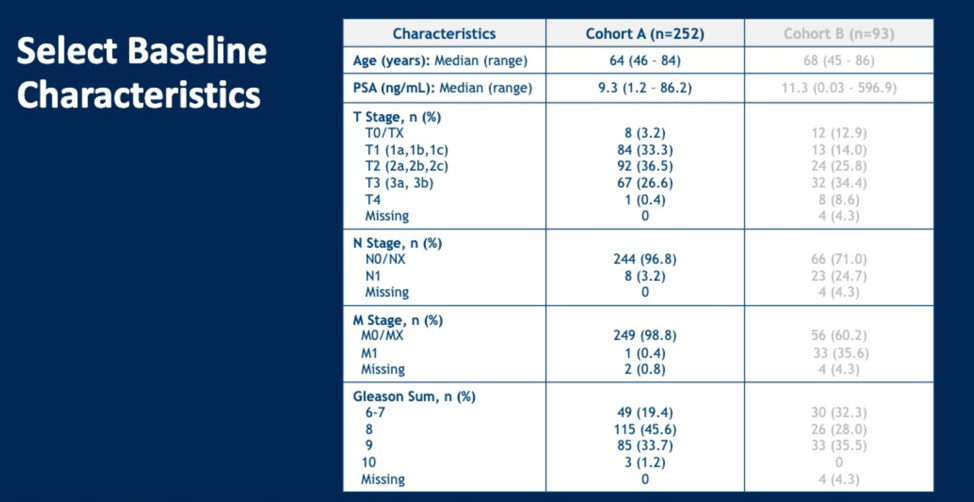
Prior to PET scan, 97% of patients had no known nodal disease, and 99% of patients did not have known metastatic disease using conventional CT or bone scan imaging. 18F-DCFPyL PET imaging identified 14.7% (37) of patients with N1 disease and 10.7% (27) of patients with M1 disease (mostly M1b), resulting in 22% (56) of patients being upstaged. The sensitivity, specificity, NPV and PPV results are shown below for lymph node positivity, broken down by central reader. True positives for lymph node disease were based on findings at lymphadenectomy. Only one metastatic lesion was biopsied.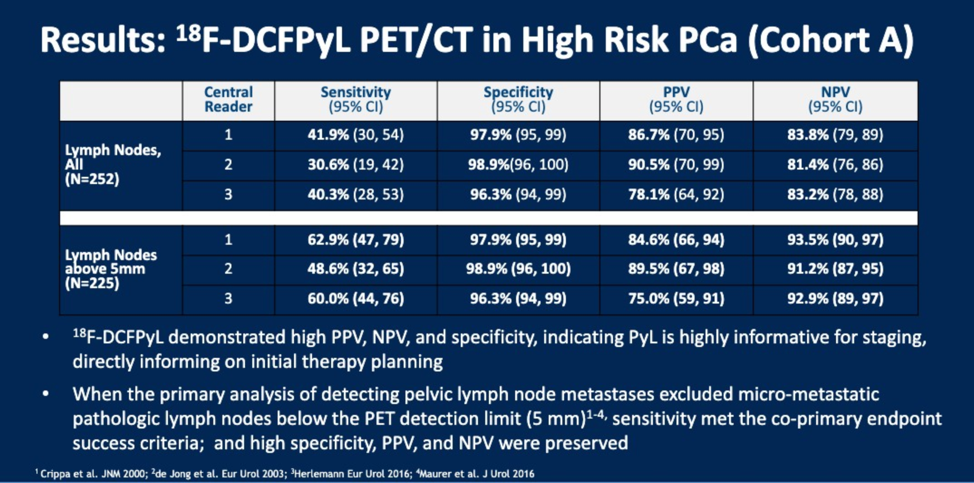
The most common drug-related adverse events were dysgeusia and headache.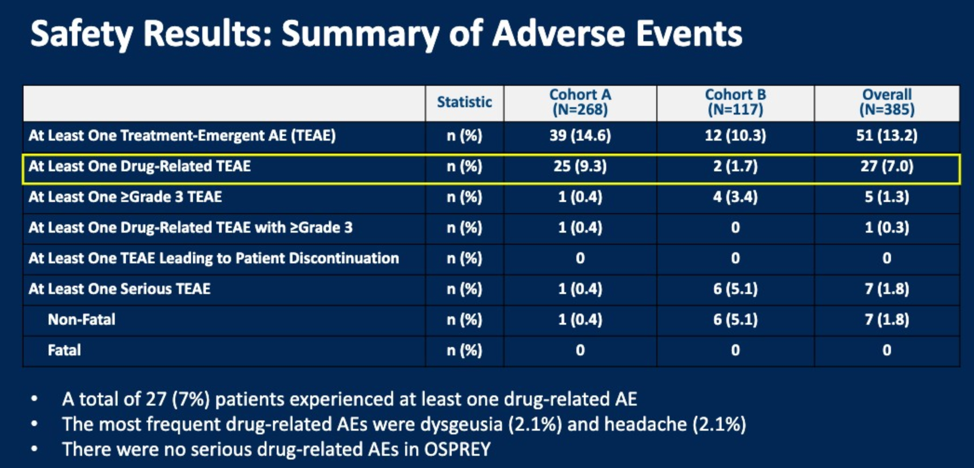
Based on these results in comparison to the prior meta-analysis of CT and MRI for staging, the authors suggest that 18F-DCFPyL improves specificity as well as the NPV and PPV of upfront disease staging.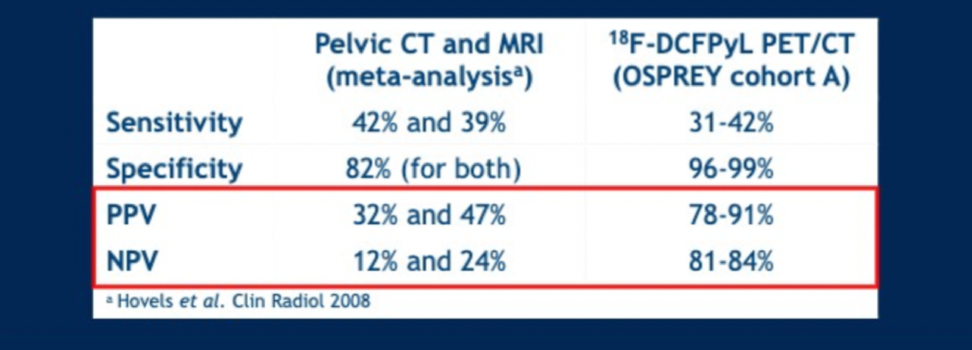
Overall, the results from this prospective, multi-center, central reader blinded study indicated that 18F-DFPyL is safe and well-tolerated in men with high-risk prostate cancer, and in this cohort improves the specificity, PPV and NPV of for detection of nodal and distant metastases compared to conventional imaging. Additional prospective trials are ongoing using 18F-DCFPyL are ongoing (CONDOR, NCT03739684, ARROW NCT03939689, others).
There are a few caveats to this study. Only 1 of the 27 presumptive metastatic lesions was biopsied and confirmed to be a true positive, so the overall performance of this test for metastatic lesions is unknown. Additionally, the impact of scans on patient management was not discussed in detail, and the impact of this modality on patient disease outcomes has yet to be determined. Finally, the ultimate utility of this PSMA-PET tracer relative to others in development (18F-rhPSMA, 18F-PSMA-1007) or relative to 68Ga PSMA-PET remains to be determined.
Presented by: Frederic Pouliot, MD, PhD, Assistant Professor, Department of Surgery, Urology Division, Laval University Hospital Center, Quebec, Canada.
Written by: Alok Tewari, MD, PhD, Medical Oncology Fellow at the Dana-Farber Cancer Institute, at the 2020 Genitourinary Cancers Symposium, ASCO GU #GU20, February 13-15, 2020, San Francisco, California


