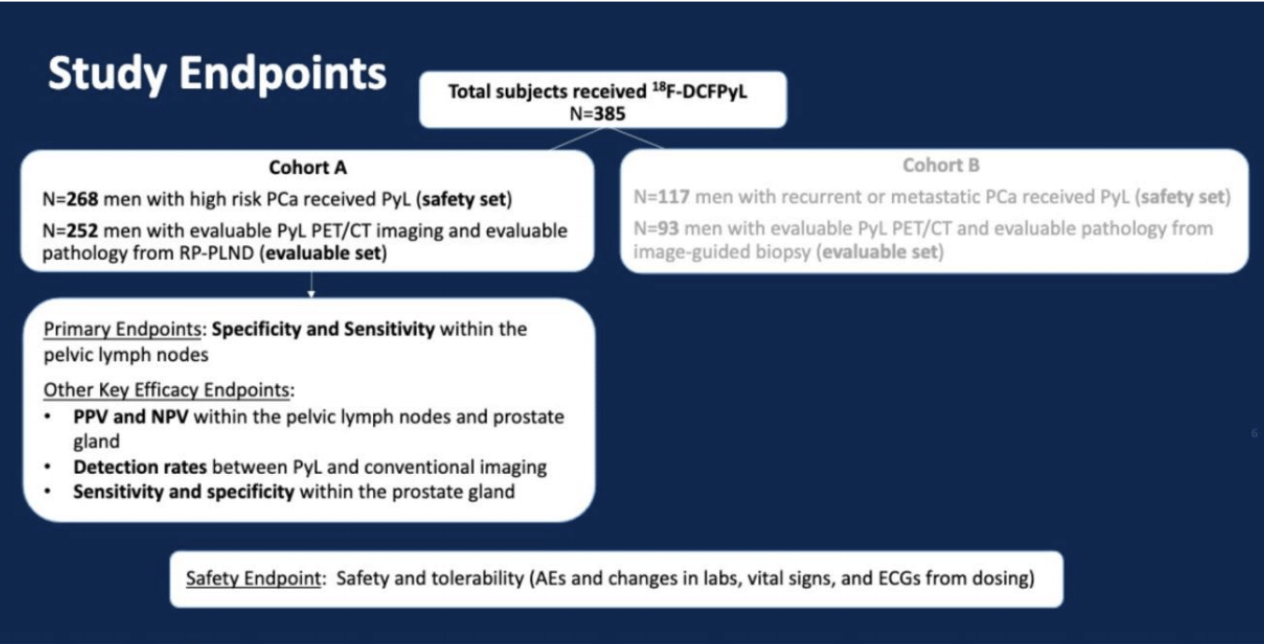
Dr. Pouliot then described the OSPREY study purpose and design. The purpose was to evaluate the diagnostic performance of 18F-DCFPyL PET/CT imaging in the initial staging of men with high-risk prostate cancer (Cohort A, n=268) and the detection of recurrent or metastatic prostate cancer (Cohort B, n=117), based on blinded central reads compared to histology as the true standard. This was a Phase 2/3, open-label, prospectively-designed, and well-controlled pivotal study conducted at ten sites in the US (n=8) and Canada (n=2). There were three central and independent PSMA PET/CT readers blinded to all clinical information and one conventional imaging central reader, again blinded to all clinical information and PSMA scans and results. 9mCi (333 MBq) of 18F-DCFPyL was administered 1-2 hours before PET/CT. Based on TNM staging, 18F-DCFPyL PET/CT detection rates, including lesion counts, were systematically analyzed: prostatic (T), pelvic LN (N), extra-pelvic LN (M1a), bone (M1b) and other visceral organs/soft tissue (M1c).
Dr. Pouliot then summarized the results from Cohort A. 18F-DCFPyL demonstrated high PPV (78.1-90.5%), NPV (81.4-83.8%), and specificity (96.3-98.9%), indicating that 18F-DCFPyL is highly informative for staging, directly informing on initial therapy planning. When the primary analysis of detecting pelvic lymph node metastases excluded micro-metastatic pathologic lymph nodes below the PET detection limit (5mm), sensitivity (48.6-62.9%) met the co-primary endpoint success criteria; and high specificity, PPV, and NPV were preserved. At study entry, >96% of patients had no known regional or distant metastatic disease (N0/NX and M0/MX)based on conventional imaging. A total of 72/268 (27%) patients had N1/M1 detected on 18F-DCFPyL PET/CT. 18F-DCFPyL PET/CT staged 39 (14.6%) patients with N1 disease and 33 (12.3%) patients with M1 disease (2 M1a, 24 M1b, and 7 M1c). He then highlighted a high-risk prostate cancer patient who had a negative bone scan, but 18F-DCFPyL PET showed multifocal osseous lesions involving the spine, ribs, pelvis, and right clavicle. He did not undergo radical prostatectomy and underwent a biopsy of 18F-DCFPyL detected bone lesion, which was confirmed to be a true positive at pathology. Dr. Pouliot then summarized the adverse events from both Cohort A and B. A total of 27 (7%) patients experienced at least one drug-related adverse event. The most frequent drug-related adverse events were dysgeusia (2.1%) and headache (2.1%). There were no serious drug-related adverse events.
Dr. Pouliot concluded his excellent talk by noting that18F-DCFPyL was safe and well-tolerated. In men with high-risk prostate cancer for metastatic disease who are a candidate for radical prostatectomy with lymphadenectomy, 18F-DCFPyL PET/CT improved clinical N and M staging despite completely blinded image reads. These results suggest the potential utility of 18F-DCFPyL PET/CT in the staging of men with newly diagnosed high-risk prostate cancer to develop optimized treatment paradigms and to directly impact patient management. There are other prospective trials with 18F-DCFPyL PET such as CONDOR, which will be presented at an upcoming meeting, and ARROW, which is in progress.
Presented by: Frederic Pouliot, MD, PhD, Assistant Professor, Department of Surgery, Urology Division, Laval University Hospital Center, Quebec, Canada.
Written by: Abhishek Srivastava, MD, Society of Urologic Oncology Fellow, Fox Chase Cancer Center, Fox Chase Cancer Center, Philadelphia, PA at the annual ASCO GU meeting 2020, San Francisco, CA. Twitter: @shekabhishe at the 2020 Genitourinary Cancers Symposium, ASCO GU #GU20, February 13-15, 2020, San Francisco, California
Written by: Abhishek Srivastava, MD, Society of Urologic Oncology Fellow, Fox Chase Cancer Center, Fox Chase Cancer Center, Philadelphia, PA at the annual ASCO GU meeting 2020, San Francisco, CA. Twitter: @shekabhishe at the 2020 Genitourinary Cancers Symposium, ASCO GU #GU20, February 13-15, 2020, San Francisco, California


