(UroToday.com) Prostate-specific membrane antigen (PSMA)-targeted imaging has rapidly gained interest and utilization in patients with prostate cancer. Recently, two approaches (68-Gallium and Piflufolastat F-18 [PYLARIFY, previously known as 18F-DCFPyL]) have gained FDA approval for prostate cancer patients with suspected recurrence based on elevated PSA. This is driven by a proven ability to better localize and determine the extent of recurrent disease, compared with conventional imaging approaches. Two trials, OSPREY and CONDOR, formed the basis of the approval of Piflufolastat F-18.
In a poster presentation at the Society of Urologic Oncology Annual Meeting, Dr. Carroll presented the correct localization rate (CLR) in patients with biochemical recurrence based on their prior treatment regimens from the CONDOR trial.
As previously presented and published, CONDOR recruited men (18 or older) with a rising PSA following definitive therapy (radical prostatectomy (RP) and/or radiation therapy (RT)) for histologically confirmed prostate cancer. Patients had to have negative or equivocal conventional imaging.
Patients received Piflufolastat F-18 (~9 mCi (333 MBq as a single dose followed by a whole-body PET/CT scan one to two hours later. The authors then assessed CLR, a novel primary endpoint based on PPV (TP/(TP+FP)), and defined it as a percentage of patients with a one-to-one correspondence between localization of at least one lesion identified on piflufolastat F 18-PET/CT and a composite standard of truth (SOT). The SOT was based on histopathology, correlative imaging, or PSA response following radiation therapy. All Piflufolastat F 18-PET/CT scans were centrally reviewed by three blinded independent readers.
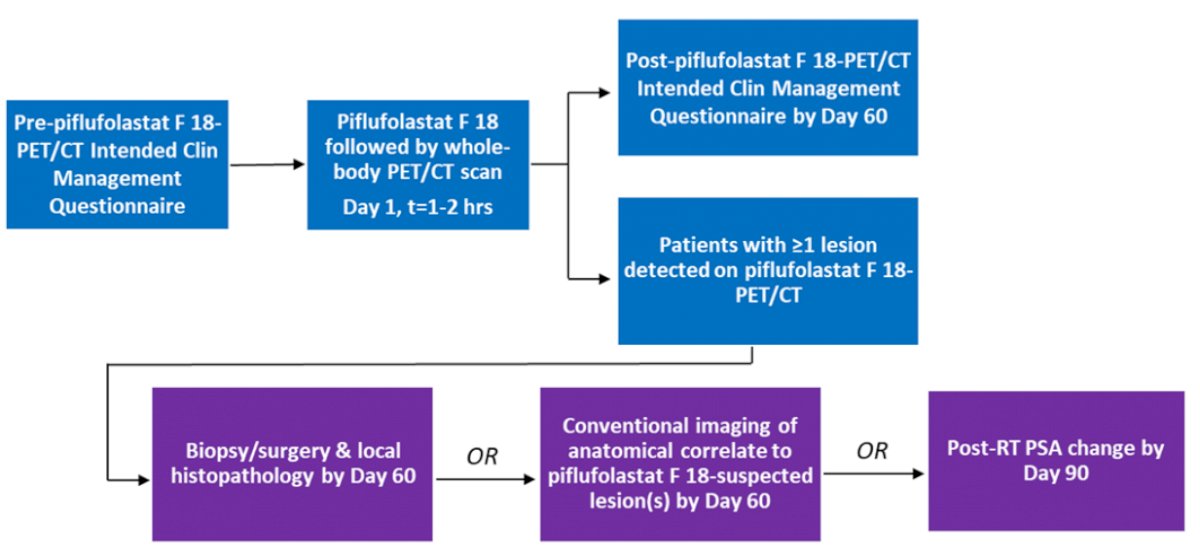
The authors included 208 men who, at the time of Piflufolastat F-18 imaging had a median PSA of 0.8 ng/mL (range: 0.17–98.45). Independent of prior treatment regimens, the overall CLR was 84.8% to 87.0% across three blinded independent readers. The CLR varied somewhat across prior primary definitive treatment regimens.

For RP only patients with no prior androgen deprivation therapy (ADT; n=97), the CLR ranged from 78.8% (26/33) to 83.9% (26/31) across the three readers. Notably, the CLR was slightly higher in patients who had received ADT as part of prior management and ranged from 90.0% to 93.8% compared to 82.4% to 83.8% in patients without prior ADT exposure
The authors, therefore, concluded that there was a consistently high CLR for patients undergoing piflufolastat F 18-PET/CT at the time of biochemical recurrence, regardless of prior definitive treatment regimens (RP, RT, or RP+RT, +/- ADT). ClinicalTrials.gov identifier NCT03739684
Presented by: Peter R. Carroll, MD, MPH, Professor, UCSF Department of Urology, San Francisco, CA

