Piflufolastat F 18-PET/CT was evaluated in men with NCCN high-risk prostate cancer scheduled to undergo radical prostatectomy with pelvic lymphadenectomy (RP-PLND) (Cohort A) and men with radiologically suspected recurrent/metastatic prostate cancer (Cohort B). A single IV dose of 9 mCi (333 MBq) of piflufolastat F 18 was administered followed by PET/CT acquisition 1-2 hours later. Piflufolastat F 18 uptake in various lesion locations, as defined by the maximum and peak SUV (SUVmax, SUVpeak), were determined by three blinded, independent central readers for each tissue (e.g., bone, lymph nodes, soft tissue). To measure SUVs, the reader placed a volume of interest on each identified lesion. SUVmax was defined as the maximum single-voxel SUV within the VOI. SUVpeak within the volume of interest was defined as the average SUV within a fixed-sized volume of interest (1 cm diameter sphere), representing the cluster with the highest average SUV.
There were 345 men that underwent piflufolastat F 18-PET/CT. Cohort B (93 evaluable patients) SUVmax and SUVpeak were significantly higher for biopsy positive (one biopsy lesion/patient) when compared to biopsy negative lesions from bone and lymph nodes:
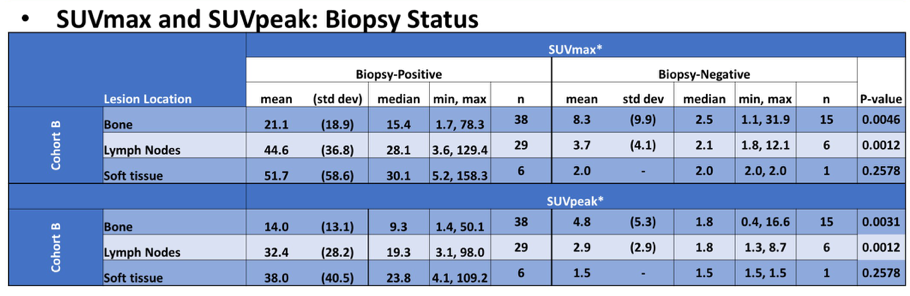
SUVpeak for biopsied bone and lymph nodes (Cohort B) appeared to increase with rising baseline PSA: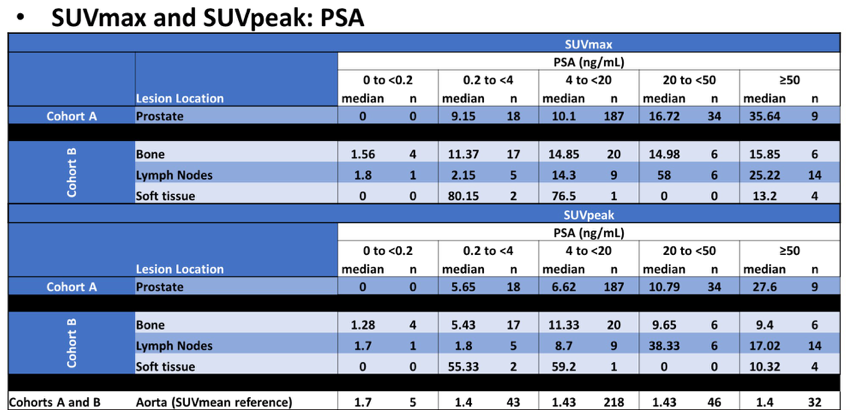
In high-risk prostate cancer patients, SUVpeak for prostate (Cohort A; 252 evaluable patients) increased with baseline PSA and were highest for Gleason Score 9-10: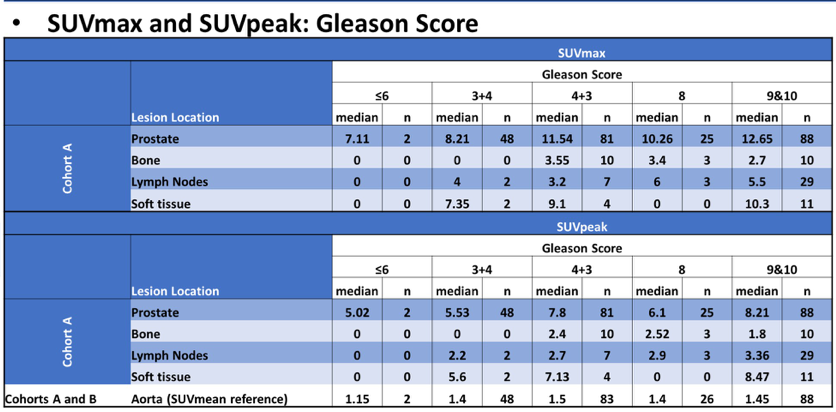
Dr. Gorin concluded his presentation of SUV results for Piflufolastat F 18-PET/CT in the OSPREY trial stratified by PSA and Gleason score with the following concluding statements:
- Piflufolastat F 18-PET/CT uptake was significantly higher in biopsy positive lesions and increased with baseline PSA
- Prostate SUVpeak was highest for Gleason Score 9-10
Presented by: Michael A. Gorin, MD, Johns Hopkins University School of Medicine, Baltimore, MD
Co-Authors: Steven P. Rowe, Kenneth J. Pienta, Peter R. Carroll, Frederic Pouliot, Stephan Probst, Lawrence Saperstein, Mark Preston, Ajjai Shivaram Alva, Akash Patnaik, Nancy Stambler, Barry A. Siegel, Michael J. Morris
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, Thurs, Feb 17 – Sat, Feb 19, 2022.
References:


