(UroToday.com) In a session on the third day of the American Society for Clinical Oncology (ASCO) Genitourinary Cancer Symposium 2022 entitled Regulating the Wild West: PET-Based Imaging in Trials and the Clinic, Dr. Shane Masters, from the Division of Imaging and Radiation Medicine at the Office of Specialty Medicine of the US Food and Drug Administration, discussed regulatory consideration for the use of PSMA-PET in clinical trials of patients with prostate cancer.
He began by highlighting the recent FDA approvals of Ga-68 gozetotide at UCLA and UCSF in December 2020 which were followed quickly by approval of piflufolastat F-18 from Progenics Pharmaceuticals in May 2021 and a Ga-68 gozetotide kit from Telix Pharmaceuticals in December 2021.
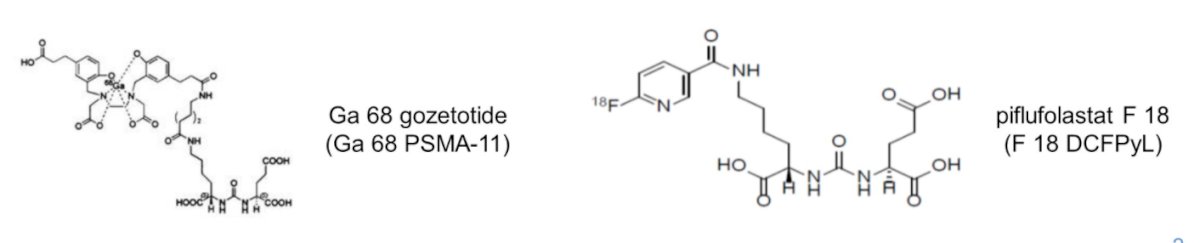
These agents have been assessed in a number of trials supporting their approval. For Ga-68 gozetotide, the PSMA-PreP and PSMA-BCR studies provided a rationale for the use of this agent in primary staging and localization of prostate cancer lesions in patients with biochemical recurrence, respectively. Similarly, for piflufolastat F-18, the OSPREY trial (cohort A) and CONDOR trial supported the role of this agent for primary staging and localization of disease in biochemical recurrence, respectively. Dr. Masters noted that, in the CONDOR trial, patients had to have negative or equivocal conventional imaging.
On the basis of these data, we may consider the PSMA-PET Drug indication. The agent is indicated for positron emission tomography (PET) of PSMA-psotive lesions in men with prostate cancer: (1) with suspected metastasis who are candidate for initial definitive therapy or (2) with suspected recurrence based on elevated serum PSA levels. However, in the context of disease monitoring, PSMA-PET has not yet been demonstrated to be a clinically meaningful endpoint to allow for regulatory submission. In part, this is due to heterogeneity – thus, Dr. Masters emphasized the importance of standardizing the scan technique and interpretation/reporting criteria. Further, there is an outstanding need for validated progression and response criteria.
In terms of the scan technique, he highlighted that there is a myriad of factors that may affect PSMA-PET imaging to some extent including the drug, radioactivity dose, mass dose, use of a diuretic, dose to scan time, anatomic imaging technique, and scan coverage. Further, there may be within and between patient differences, emphasizing the importance of serial comparison to a baseline scan.
In terms of interpretation and reporting criteria, Dr. Masters highlighted the importance of lesion definitions, region definitions, reader number, and training. There are currently a number of proposed criteria (including PSMA-RADS, PROMISE, E-PSMA, and Pro-PET) with an outstanding need for standardization.
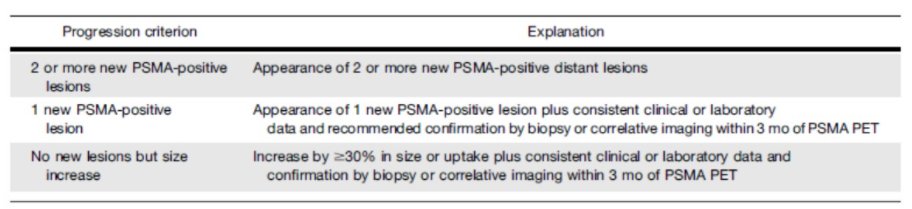
In terms of assessing for disease progression, the evidence of a new lesion is one relevant criterion. However, even in this context, we need to consider the number of new lesions, their location (local versus distant), and the clinical significance of lesions that are PSMA-PET positive but negative on conventional imaging. As Dr. Morgans had done earlier in the session, he highlighted recently proposed PSMA-PET Progression criteria.
In terms of treatment response assessment, this is typically assessed based on a decreased uptake, measured by SUV. Recently, the EAU/EANM Consensus Panel proposed criteria to categorize treatment response into complete response, partial response, and stable disease based on PSMA-PET imaging.

Moving towards a more standardized approach, Dr. Masters highlighted semiquantitative metrics in the interpretation of PSMA-PET. He emphasized that measures may be subject to many sources of variability related to the scan, the measurement, and day-to-day variation. Further, there may be both pathophysiologic and treatment-induced changes in PSMA expression. Thus, this may result in an interaction between PSMA expression as a response metric and as a predictive or prognostic biomarker.
Presented by: Shane C. Masters, MD, PhD, U.S. Food and Drug Administration

