(UroToday.com) The 2024 IBCN annual meeting included a session on novel therapies and outcome measures in clinical trials, featuring a presentation by Dr. Colin Dinney discussing results from a comprehensive analysis from BOND-003 assessing translational correlates using urinary genomic disease burden to assess cretostimogene grenadenorepvec. Cretostimogene grenadenorepvec is a serotype-5 oncolytic adenovirus designed to selectively replicate in bladder cancer cells with alterations in the Retinoblastoma-E2F pathway. Additionally, the virus is engineered to express the GM-CSF transgene, resulting in a potent oncolytic immunotherapy mechanism of action. Pretreatment is required with the detergent dodecyl maltoside (DDM) to enhance gene transfer across the urothelium. The presumed mechanisms of action include lysis of Rb-deficient or E2F expressing tumor cells and immune activation by GM-CSF and neoantigens.
Urinary genomic disease burden profiling uses next-generation sequencing to identify mutations associated with bladder cancer. It has been shown in prior studies to be associated with bladder cancer recurrences and responses to therapy. In the ongoing BOND-003 trial, in patients with BCG-unresponsive NMIBC, cretostimogene monotherapy demonstrated complete response rates of 75%, at any time. In this translational analysis, Dr. Dinney and colleagues evaluated the associations between cretostimogene treatment and urinary genomic disease burden.
BOND-003 was a phase 3, single arm, open-label trial of intravesical cretostimogene monotherapy. Patients were BCG-unresponsive NMIBC with CIS +/- Ta/T1, with all Ta/T1 disease having been resected prior to treatment. There was a mandatory biopsy at 12 month assessment based on the trial protocol:
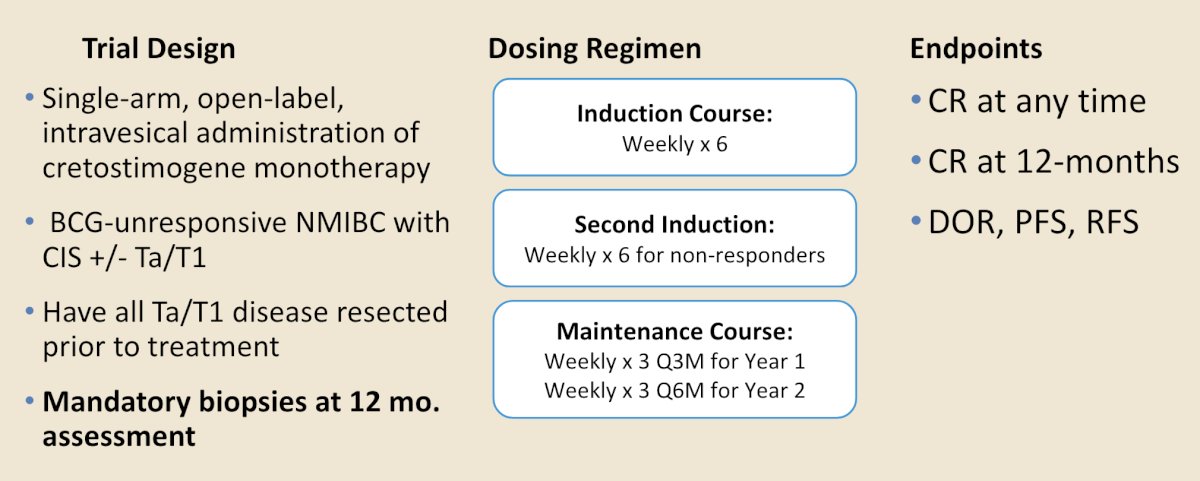
Presented at AUA 2024, in BOND-003, cretostimogene monotherapy showed durable responses, with a 3 month complete response rate of 68% and an anytime complete response rate of 76%. Notably, 54% of re-induced patients converted to complete response. The 12-month cystectomy free rate was 92%, 12 month progression free survival rate was 97%, and cretostimogene was well tolerated with no grade 3-5 treatment related adverse events, and no patients discontinued treatment due to a treatment related adverse event.
So, how can we improve intravesical gene therapy? Dr. Dinney noted the following points:
- Improve patient selection
- Identify risk features or biomarkers that predict response or resistance
- Improve complete response rates by selecting likely responders for treatment
- Support stratification of control and intervention arms to allow for treatment escalation and de-escalation
UroAmp is a Convergent Genomics genetic platform that quantifies mutations and DNA alterations from urine cell free DNA. It utilizes deep NGS of a 60 gene bladder cancer specific panel, and low pass whole genome sequencing to identify aneuploidy. UroAmp also quantifies minimal residual disease by the genomic disease burden, which is a percentile ranking of the variant allele frequency from a sample relative to its percentile ranking within the established training set. Each of the samples in this study was ranked against that distribution to obtain the genomic disease burden percentile rank to characterize the tumor as high risk or low risk for recurrence.
All patients who received at least one dose of cretostimogene and had urinary genomic disease burden testing with UroAmp were included. Urine samples were collected at baseline and follow-up time points after treatment. Pre-treatment baseline mutation profiles were available for 64 patients, and to date, post-treatment profiles have been analyzed for 51 patients at 3 months and 35 patients at 6 months. The cumulative mutation counts using the Convergent Genomics UroAmp platform are highlighted as follows:
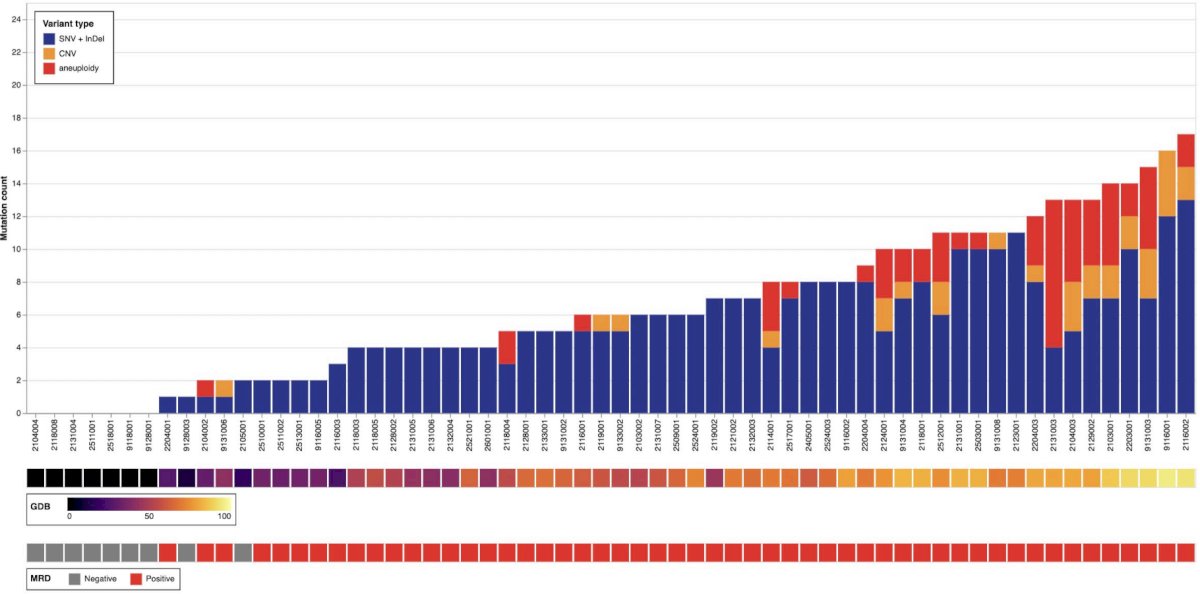
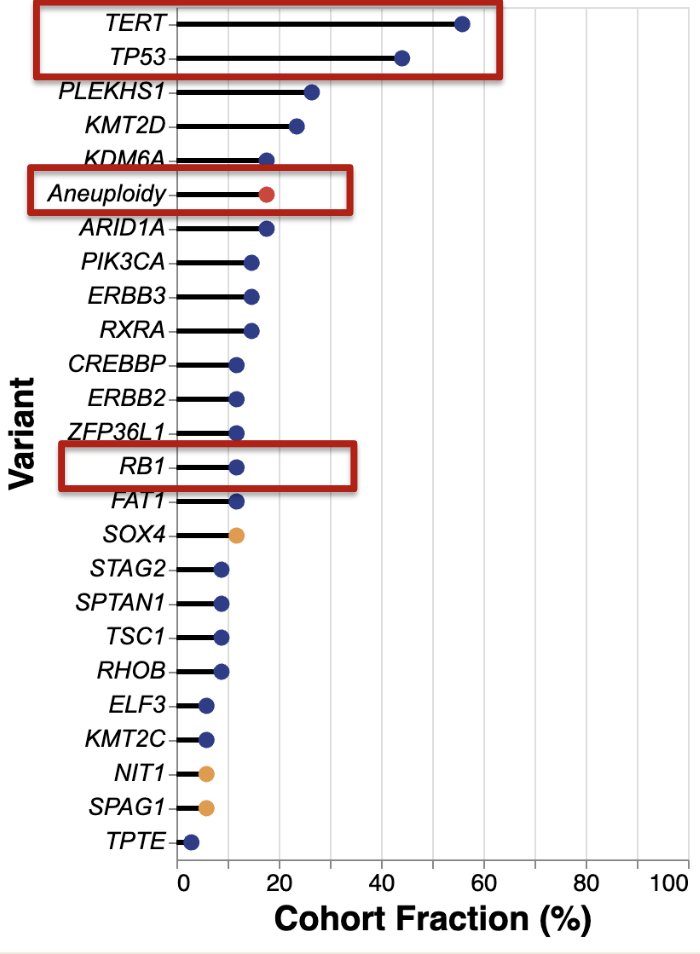
The most frequent variants in BCG unresponsive NMIBC were found in TERT, p53, genes coding for chromatin modifying enzymes and proliferation pathways. There was a prominent subset of aneuploidy positive tumors that represent higher risk subtypes of CIS sharing a genetic signature with muscle invasive bladder cancer. Rb mutations were relatively rare:
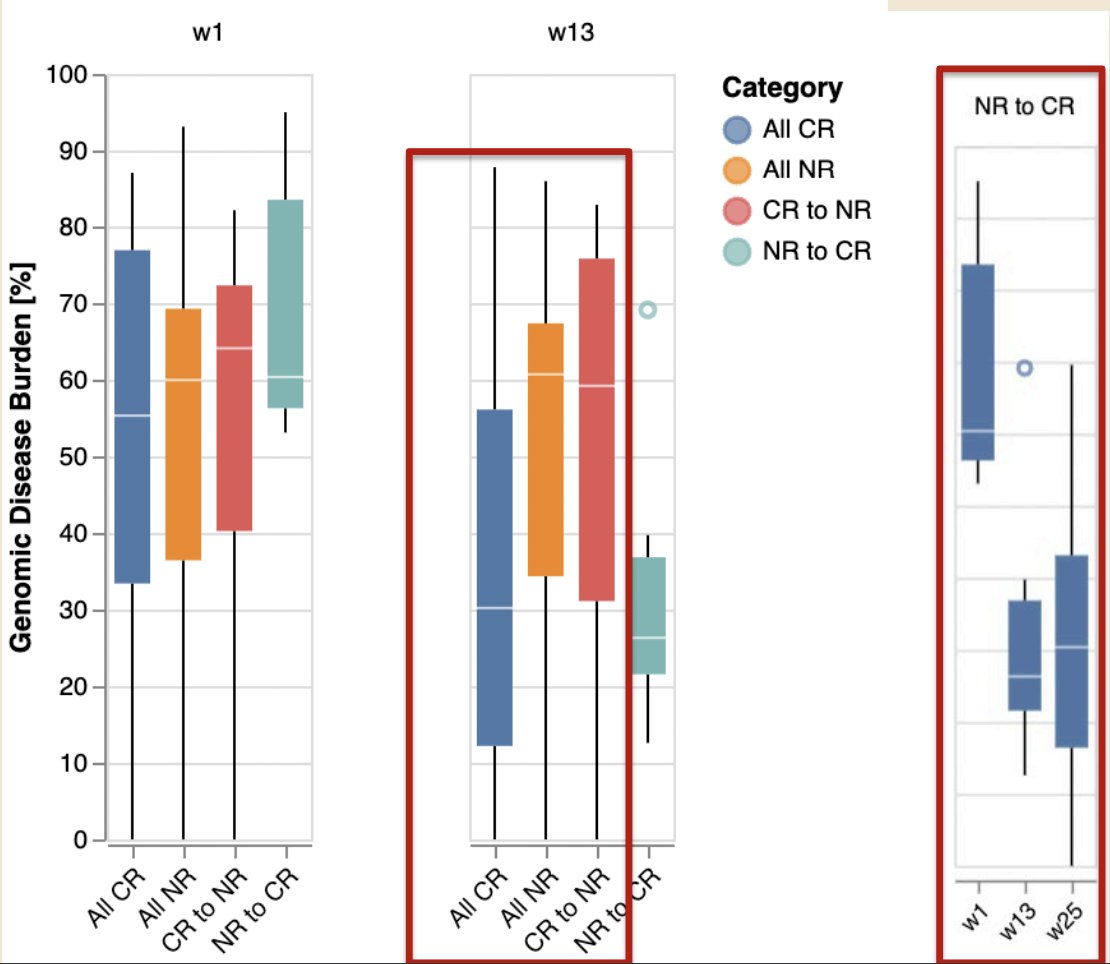
The baseline genomic disease burden for patients enrolled in BOND-003 is summarized below:
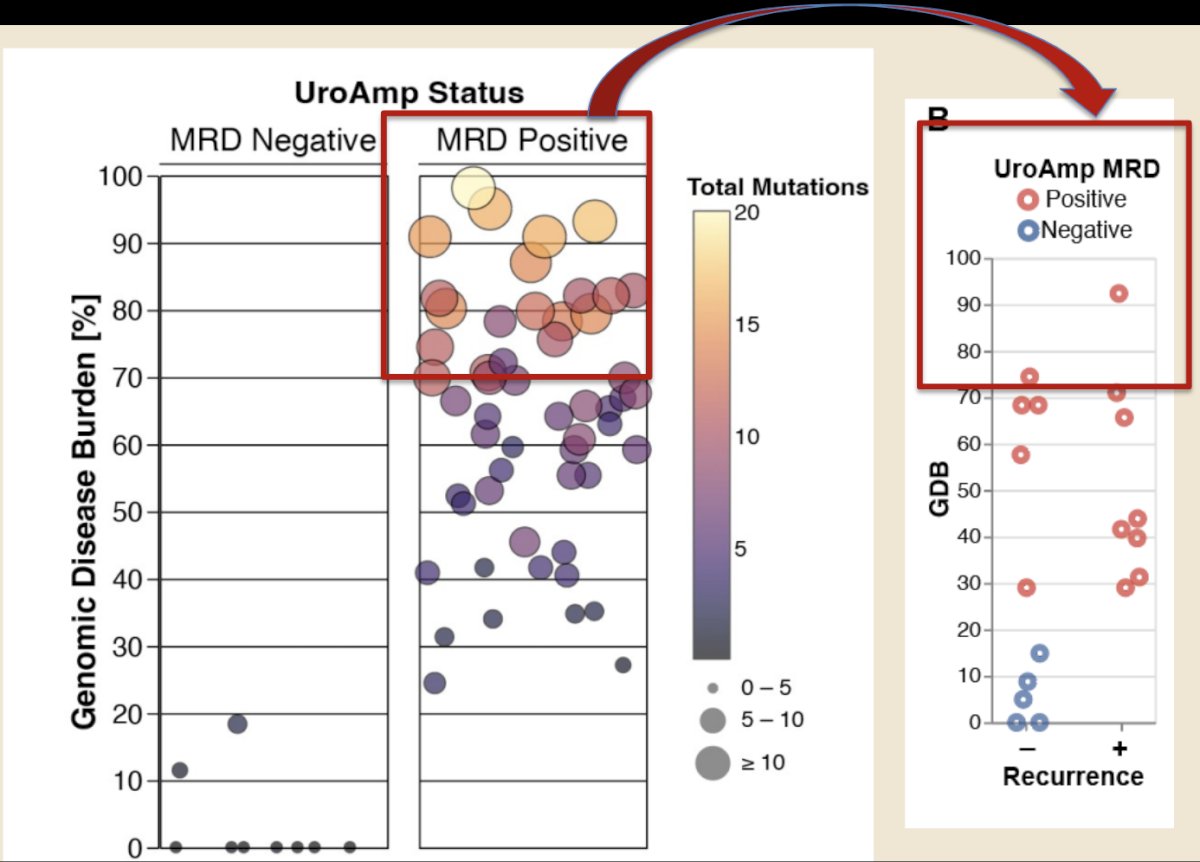
Lower genomic disease burden was seen in patients achieving a complete response at 3 months, with a dramatic reduction in genomic disease burden in re-induced patients who achieved a complete response at 6 months. As expected, changes in the variant allele frequency, which define the genomic disease burden, paralleled the changes in the genomic disease burden:
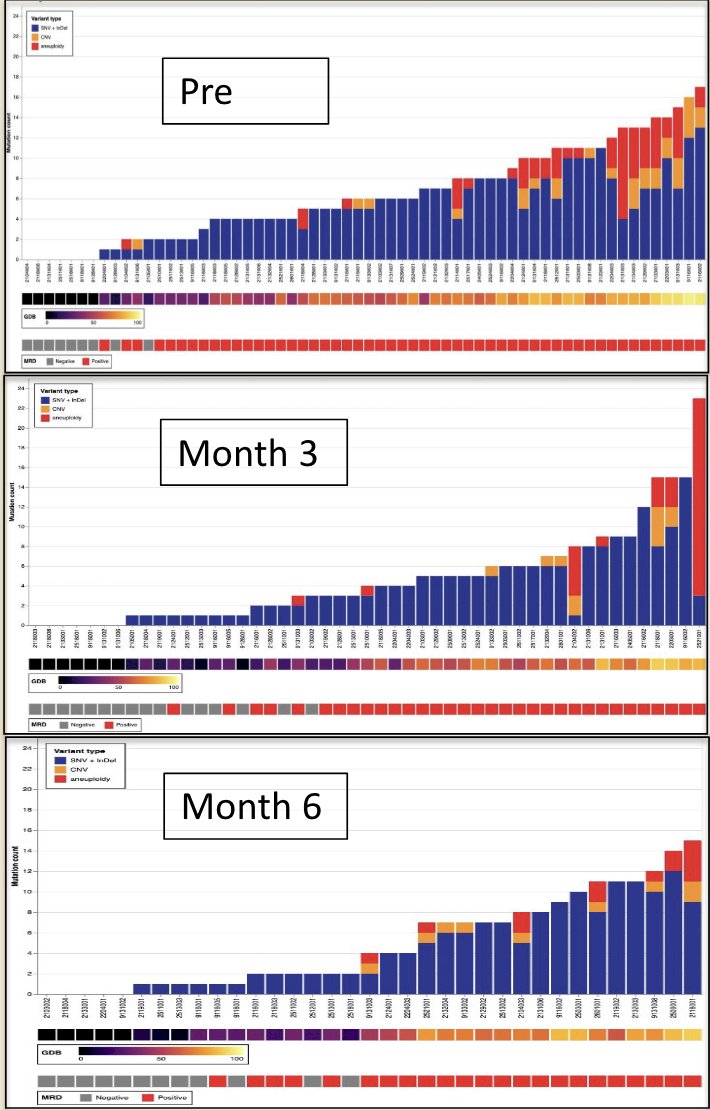
Robust genomic disease burden response to cretostimogene was characterized by a reduction in the prevalence of tumors with aneuploidy and altered ERBB2, TP53, and RB1. This molecular response suggests that cretostimogene reduces the risk of disease progression (longitudinal reduction in aneuploidy is highlighted in red, along with key mutations):
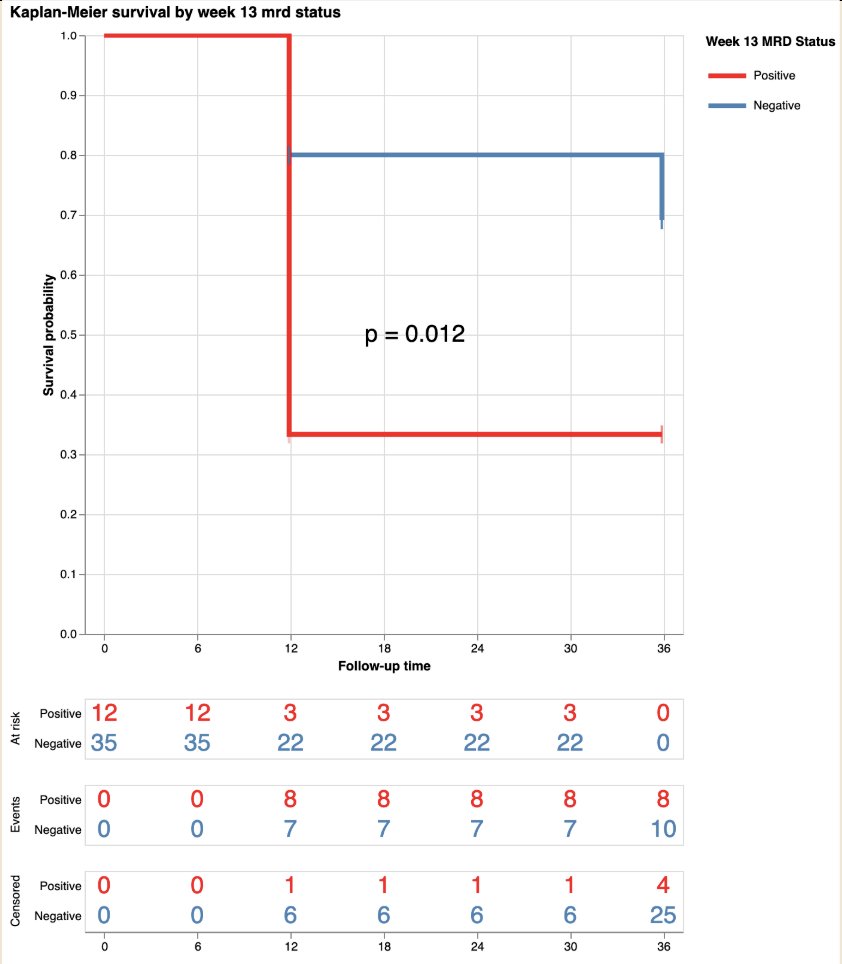
Of note, the 3 month UroAmp signature predicted 12 month recurrence free survival, with 80% recurrence free survival for low risk and 33% for high risk at 3 months (p = 0.012):
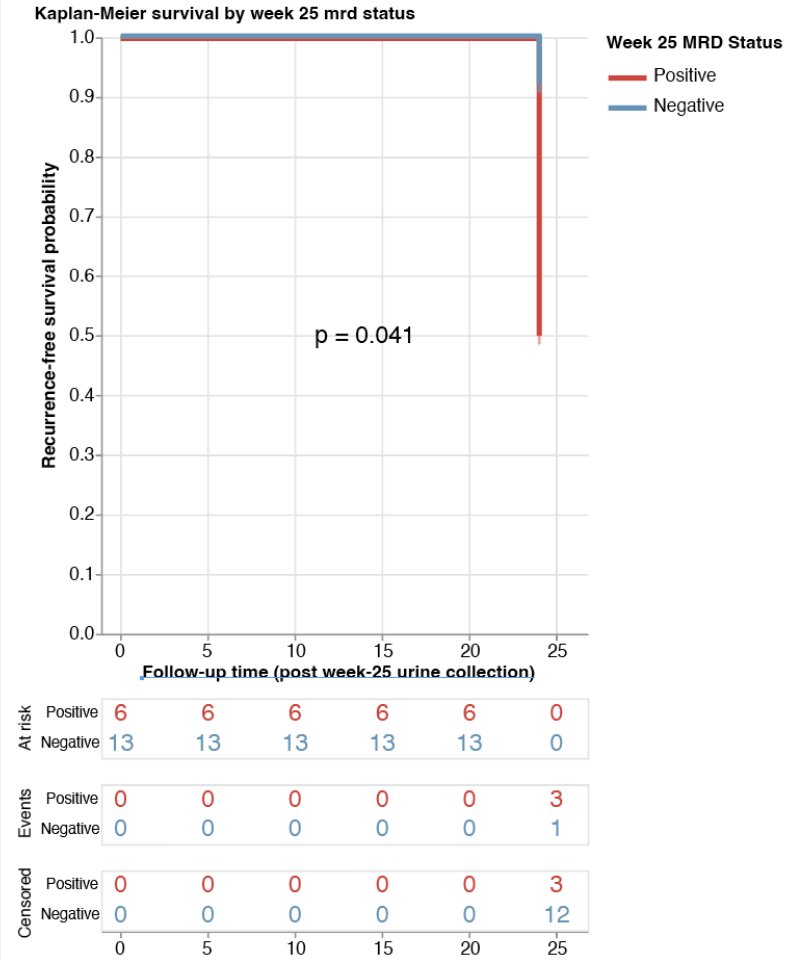
Low risk patients include patients who maintained a complete response from 3 months and those who achieved a complete response following re-induction. There was 1/13 low risk (8%) and 3/6 high risk (50%) at 6 months that had a documented recurrence at 12 months (p = 0.041). Thus, longitudinal changes in genomic disease burden are informative to clinical outcomes:
Dr. Dinney concluded his presentation by discussing results from a comprehensive analysis from BOND-003 assessing translational correlates using urinary genomic disease burden to assess cretostimogene grenadenorepvec with the following take-home points:
- Cell free urine DNA profiling with the UroAmp platform enables quantitative assessment of the clinical response to cretostimogene
- UroAmp stratified patients treated with cretostimogene as low risk or high risk and predicted durable 12 month recurrence free survival
- The notable reduction in variant allele frequency and genomic disease burden in the 54% of patients who were salvaged by re-induction reinforces the value of re-induction
- Longitudinal molecular disease burden assessment may be able to support the stratification of control and intervention arms in future clinical trials
Presented by: Colin Dinney, MD, Anderson Cancer Center, Houston, TX
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 International Bladder Cancer Network (IBCN) Annual Meeting, Bern, Switzerland, Thurs, Sept 19 – Sat, Sept 21, 2024


