(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium includes a urothelial carcinoma session featuring trials in progress and a presentation by Dr. Sia Daneshmand discussing the trial design of ABLE-41 assessing nadofaragene firadenovec-vncg early use and outcomes in a real-world setting in the United States. BCG is the first line standard of care for patients with non–muscle-invasive bladder cancer (NMIBC), however approximately one-third of patients initially responding to BCG experience recurrence and/or progression in the first year after treatment.1 Thus, local, effective, bladder-preserving options are needed for patients with BCG-unresponsive NMIBC.
Nadofaragene firadenovec-vncg is the first FDA-approved intravesical gene therapy for treatment of high-risk BCG-unresponsive NMIBC with carcinoma in situ (CIS) ± papillary tumors. In a single-arm, multicenter, open-label, repeat-dose, phase 3 study, 53.4% of patients (55 of 103) with CIS ± high-grade Ta/T1 BCG-unresponsive NMIBC achieved complete response 3 months after the first instillation of nadofaragene firadenovec.2 The safety profile of nadofaragene firadenovec was manageable, with 103 (66%) patients reporting mild (grade 1/2) study drug-related adverse events. Six (3.8%) patients had grade 3-5 study drug-related adverse events. ABLE-41 is an observational study evaluating the effectiveness, overall experiences, patterns of use, and safety in patients treated with nadofaragene firadenovec in a United States real-world setting.
This non-interventional study includes approximately 50 urology sites in the United States, with anticipated enrollment of 200 patients in 16 months. Adults ≥18 years with prescribed and scheduled treatment with nadofaragene firadenovec per physician discretion or those who received their first instillation of nadofaragene firadenovec per physician discretion after September 5, 2023, but before site activation are eligible to enroll. The study design for ABLE-41 is as follows:
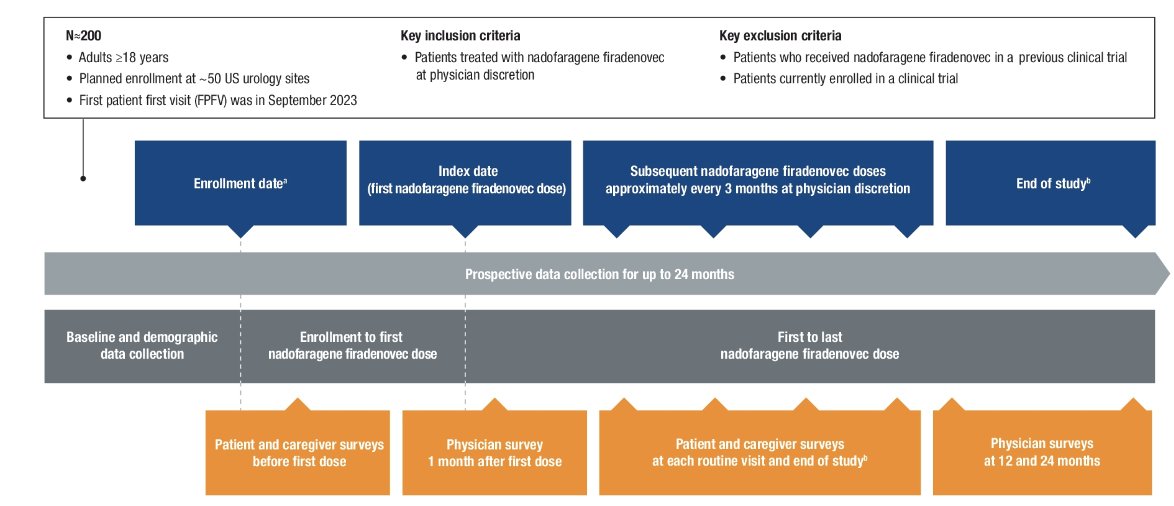
The primary objective is to assess the effectiveness of nadofaragene firadenovec measured as the complete response rate as determined by the investigator. Secondary outcomes include:
- Nadofaragene firadenovec patterns of use
- Duration of complete response
- Recurrence-free survival
- Cystectomy-free survival
- Progression-free survival
- Overall survival
- Bladder cancer–specific mortality
- Patient, caregiver and physician experiences
- Adjunctive use of molecular markers
- Safety
Patient and caregiver experiences will be assessed using the respective EuroQol 5 Dimension 5 Level questionnaire and Work Productivity and Activity Impairment questionnaire, adapted for caregiving. Patients and caregivers will be surveyed before all nadofaragene firadenovec administrations, and physicians will be surveyed 1, 12, and 24 months after first patient first instillation.
All adverse event data will be collected starting from the index date. The estimated follow-up period is 24 months, until study discontinuation, or withdrawal. Final results from this large, prospective, multi-institutional, real-world registry providing early use and outcomes of nadofaragene firadenovec are expected in December 2026.
Clinical trial information: NCT06026332.
Presented by: Siamak Daneshmand, MD, USC/Norris Comprehensive Cancer Center, University of Southern California, Los Angeles, CA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
Related Content: Real-World Use of Nadofaragene Firadenovec - Siamak Daneshmand
References:
- Sylvester RJ, Brausi MA, Kirkels WJ, et al. Long-term efficacy results of EORTC genito-urinary group randomized phase 3 study 30911 comparing intravesical instillations of epirubicin, bacillus Calmette-Guerin, and bacillus Calmette-Guerin plus isoniazid in patients with intermediate- and high-risk stage Ta T1 urothelial carcinoma of the bladder. Eur Urol. 2010 May;57(5):766-73.
- Boorjian SA, Alemozaffar M, Konety BR, et al. Intravesical nadofaragene firadenovec gene therapy for BCG-unresponsive non-muscle-invasive bladder cancer: A single-arm, open-label, repeat-dose clinical trial. Lancet Oncol. 2020 Nov 27:S1470-2045(20)30540-4.


