(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between January 25th and 27th was host to a prostate cancer poster session. Dr. Stephen Freedland presented the results of an EMBARK post-hoc analysis evaluating enzalutamide combination treatment suspension in men with high-risk biochemically recurrent prostate cancer.
EMBARK is a three-arm, randomized phase 3 trial that enrolled prostate cancer patients with high-risk biochemical recurrence, a PSA doubling time of ≤9 months, and a PSA ≥1 ng/ml after radical prostatectomy or ≥2 ng/ml above nadir following primary EBRT. Patients had no evidence of metastasis on conventional imaging. Patients were randomly assigned, in a 1:1:1 ratio, to receive (n=1,068):
- Enzalutamide (160 mg) daily plus leuprolide every 12 weeks (combination group; n=355)
- Placebo plus leuprolide (leuprolide-alone group; n=358)
- Enzalutamide monotherapy (monotherapy group; n=355).
PSA was assessed at 36 weeks, and if patients had:
- PSA<0.2 Treatment was suspended at week 37 and PSA monitored with treatment reinitiated if PSA rose again to ≥2 ng/mL (if primary radical prostatectomy) or ≥5 ng/mL (if no primary radical prostatectomy)
- PSA>0.2 Treatment was continued
The primary endpoint was metastasis-free survival (MFS), assessed via blinded independent central review (BICR), in the enzalutamide + leuprolide (combination) versus leuprolide arms only.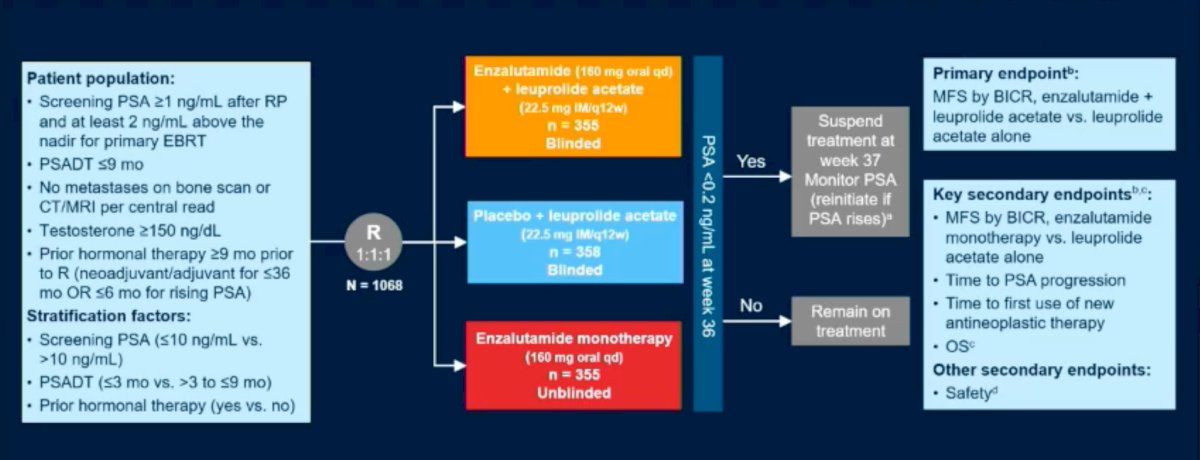
With respect to the primary outcome of MFS, enzalutamide plus leuprolide was superior to leuprolide alone (HR: 0.42, 95% CI: 0.30 – 0.61; p<0.001); enzalutamide monotherapy was also superior to leuprolide alone (HR: 0.63; 95% CI: 0.46 – 0.87; p=0.005).1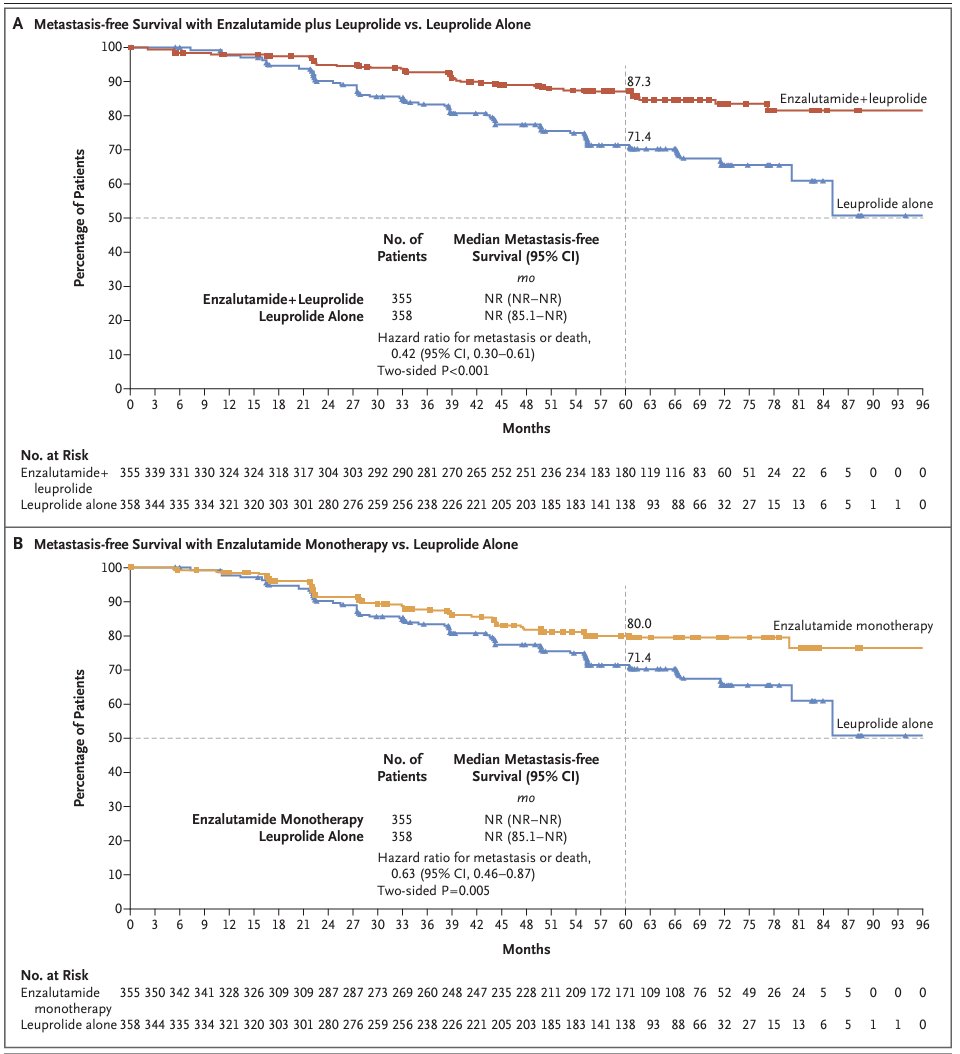
Notably, treatment was suspended at week 37 in 321 (91%) patients in the enzalutamide combination arm and 240 (68%) in the leuprolide alone arm, based on PSA response. The objective of this analysis was to report the outcomes in the enzalutamide combination versus leuprolide alone arms, by treatment suspension status.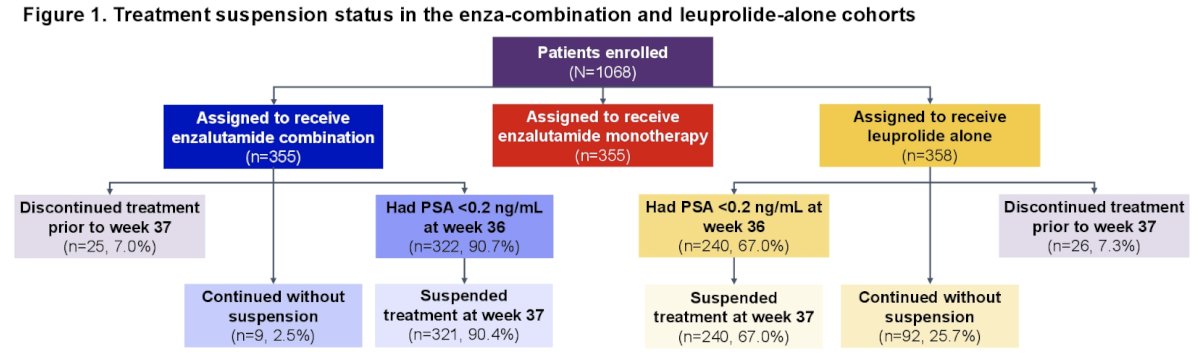
The 3-year MFS rates in the suspension groups were as follows:
- Enzalutamide combination: 94.4% (95% CI: 91.2% - 96.5%)
- Leuprolide alone: 90% (85.3 – 93.2%)
- MFS in the suspension group was improved with enzalutamide combination versus leuprolide alone (HR: 0.47, 95% CI 0.31 – 0.72, p=0.0003)

The 3-year MFS rates in the no suspension groups were as follows:
- Enzalutamide combination: 76.2% (33.2 – 93.5%)
- Leuprolide alone: 67% (55.4 – 76.1%)
- No difference in MFS was observed in the no suspension group (HR: 0.72, 95% CI: 0.23 – 2.30, p=0.58)
- Sample size was small for enzalutamide combination (n=9).
- No difference in MFS was observed in the no suspension group (HR: 0.72, 95% CI: 0.23 – 2.30, p=0.58)
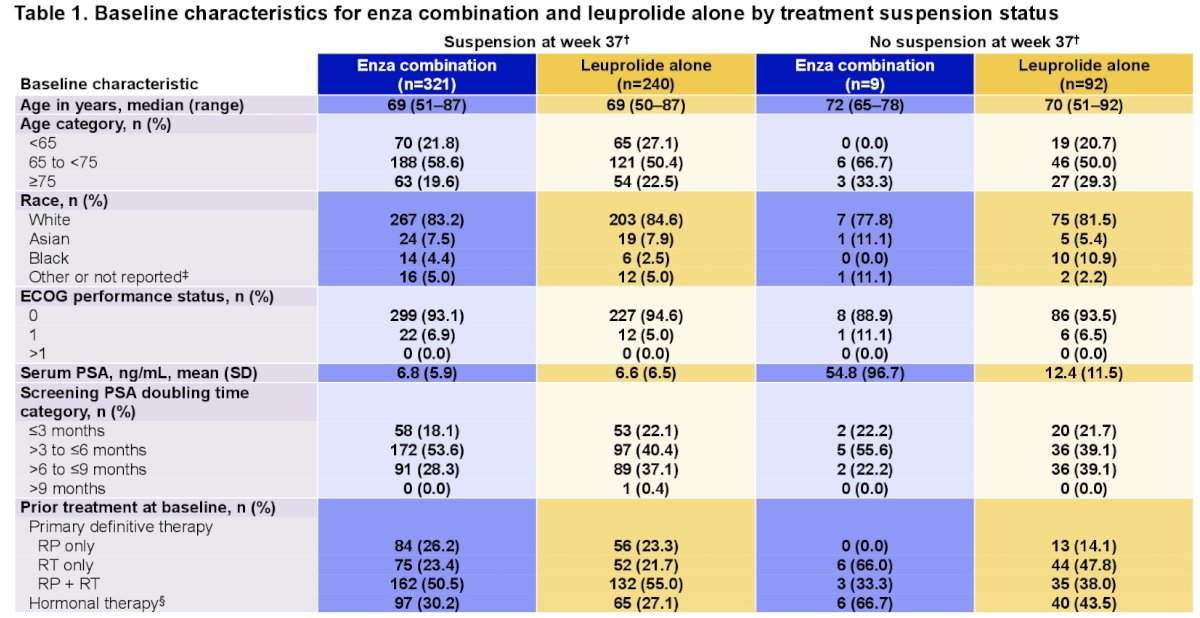
Dr. Freedland noted that compared to no suspension, a higher proportion of patients in the suspension group received prior radical prostatectomy, and a lower proportion received prior radiotherapy.
Two years after suspension, 16.8% (95% CI: 12.9 – 21.4%) of enzalutamide combination patients and 9.6% (95% CI: 6.2 – 14.0%) of leuprolide alone patients had undetectable PSA levels (p=0.0089).
Dr. Freedland concluded that MFS was improved with enzalutamide combination, compared to leuprolide alone, for patients with biochemical recurrence who suspended treatment. No difference in MFS was observed in the no suspension group, but firm conclusions were precluded by the limited number of patients who did not suspend treatment with enzalutamide combination. Patients who suspended treatment had higher proportions of prior radical prostatectomy, compared to those who did not suspend treatment. Patients who had received enzalutamide combination prior to treatment suspension were more likely to have undetectable PSA levels two years thereafter, compared to those who received leuprolide alone.
Presented by: Stephen Freedland, MD, Professor, Department of Urology, Cedars Sinai Hospital, Los Angeles, CA
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
References:

