(UroToday.com) The 2024 ESMO annual meeting included a session on prostate cancer, featuring a presentation by Dr. Neal Shore discussing a post hoc analysis from EMBARK focusing on age.
Patients diagnosed with prostate cancer aged >70 years are at greater risk of prostate cancer-related death versus patients diagnosed aged ≤70 years. Moreover, subgroup analyses of data from clinical trials of androgen receptor pathway inhibitors in prostate cancer have shown mixed results regarding the role of age on treatment effect. In EMBARK, enzalutamide ± leuprolide significantly prolonged metastasis-free survival by blinded independent central review vs leuprolide alone in patients with prostate cancer and high-risk biochemical recurrence.1 At ESMO 2024, Dr. Shore and colleagues presented a post hoc analysis of EMBARK outcomes by age.
EMBARK, a phase 3 trial (NCT02319837), included patients after local therapy with high-risk biochemical recurrence: PSA doubling time ≤9 months and PSA ≥2 ng/mL above nadir post radiotherapy or ≥1 ng/mL after radical prostatectomy ± postoperative radiotherapy. Patients were randomized (1:1:1) to enzalutamide + leuprolide, leuprolide alone, or enzalutamide monotherapy. A post hoc analysis of metastasis-free survival and safety by median age in EMBARK was conducted (<70 vs ≥70 years).
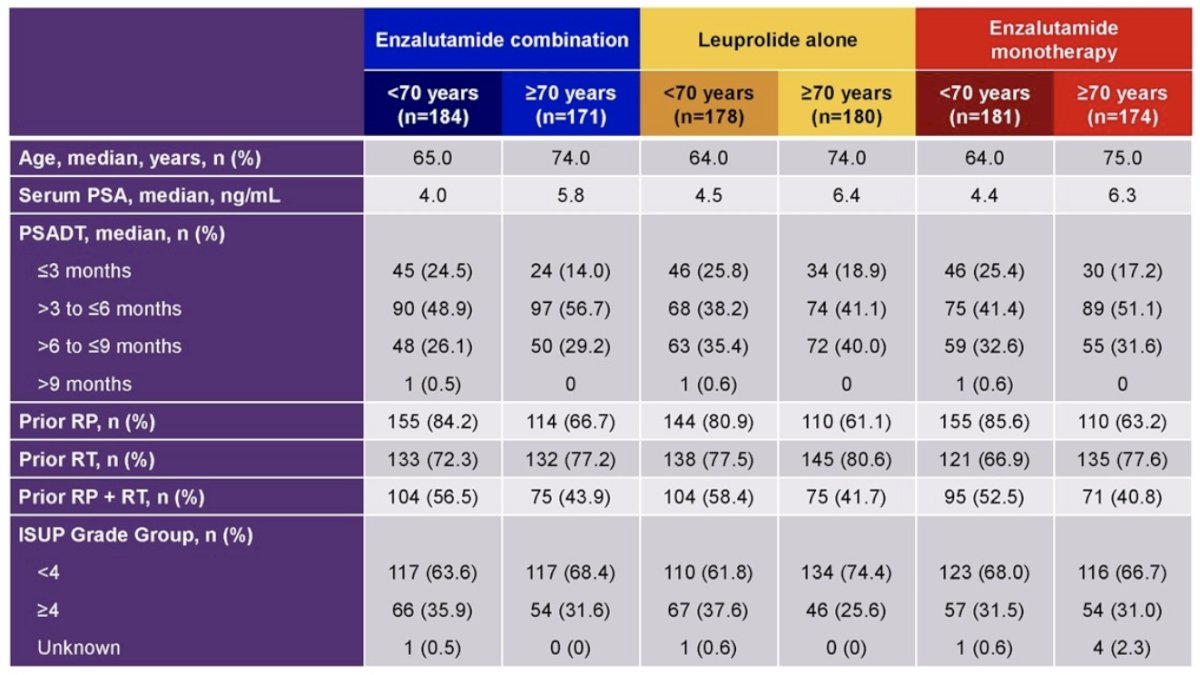
Overall, 543 patients (50.8%) were aged <70 years and 525 patients (49.2%) were ≥70 years. Patients aged <70 years had lower baseline PSA, a higher Gleason score group, and more prior radical prostatectomy versus patients aged ≥70 years across treatment groups:
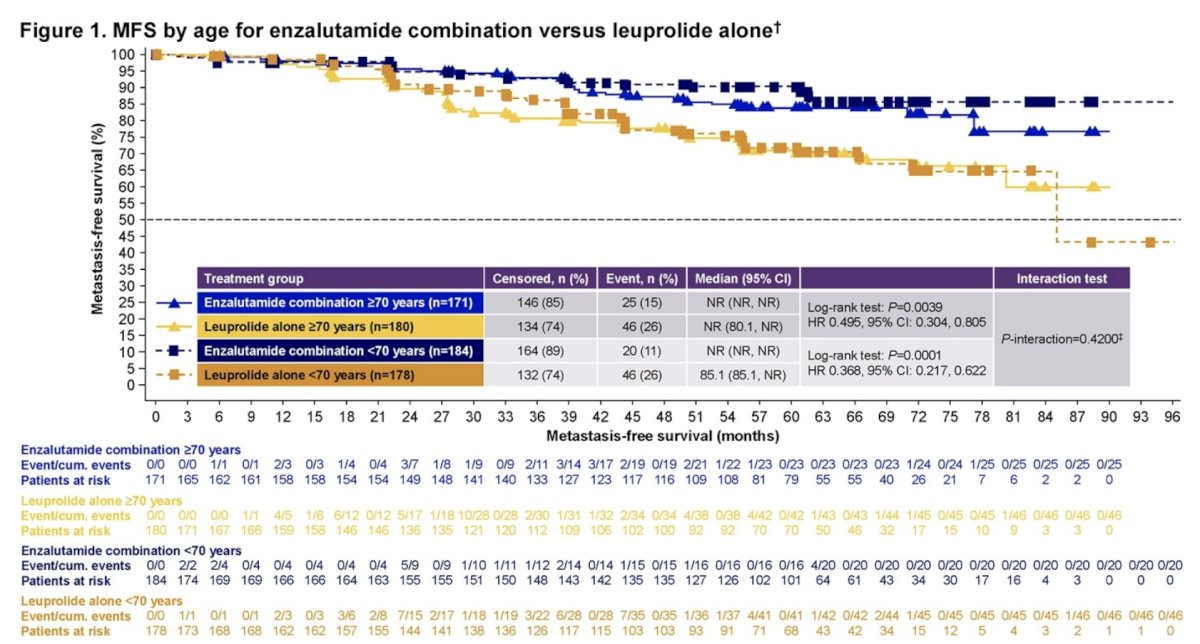
Compared with leuprolide alone, enzalutamide + leuprolide improved metastasis-free survival in both age groups:
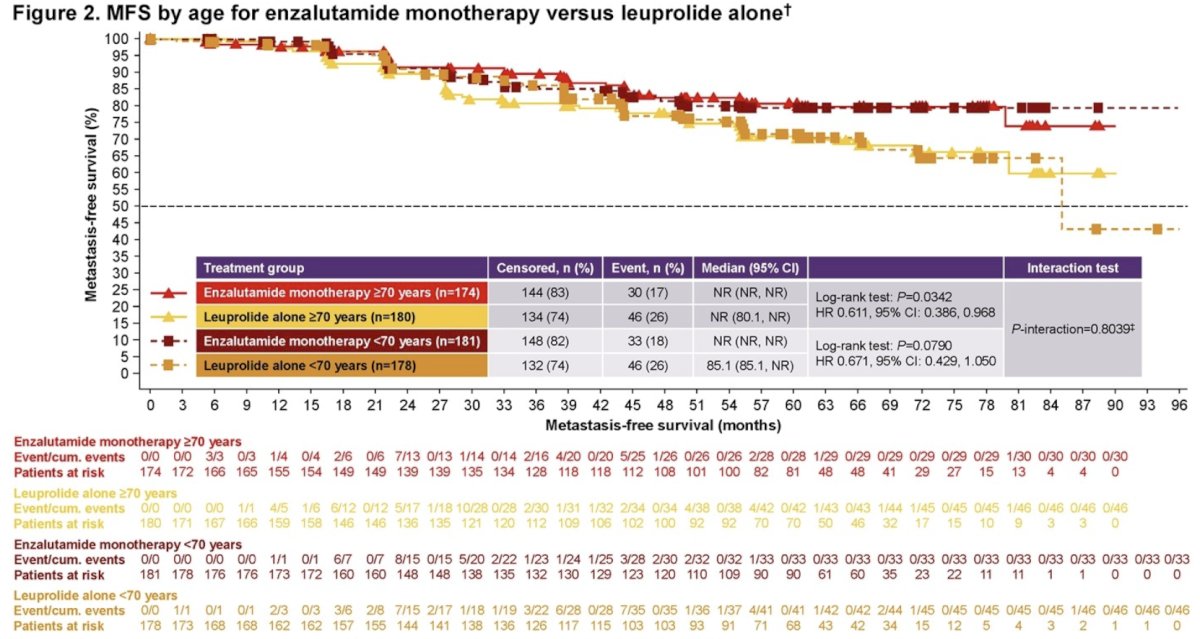
Similar trends were seen with enzalutamide monotherapy:
Treatment effect in metastasis-free survival was not statistically different between age groups for both treatment group comparisons. Overall, median treatment suspension duration was similar for patients aged <70 and ≥70 years for enzalutamide + leuprolide and enzalutamide monotherapy, but shorter for patients <70 vs ≥70 years for leuprolide alone. Serious adverse events were more common in patients aged ≥70 years vs <70 years for enzalutamide + leuprolide (45.0% vs 25.3%), leuprolide alone (36.2% vs 27.1%), and enzalutamide monotherapy (43.9% vs 30.4%):
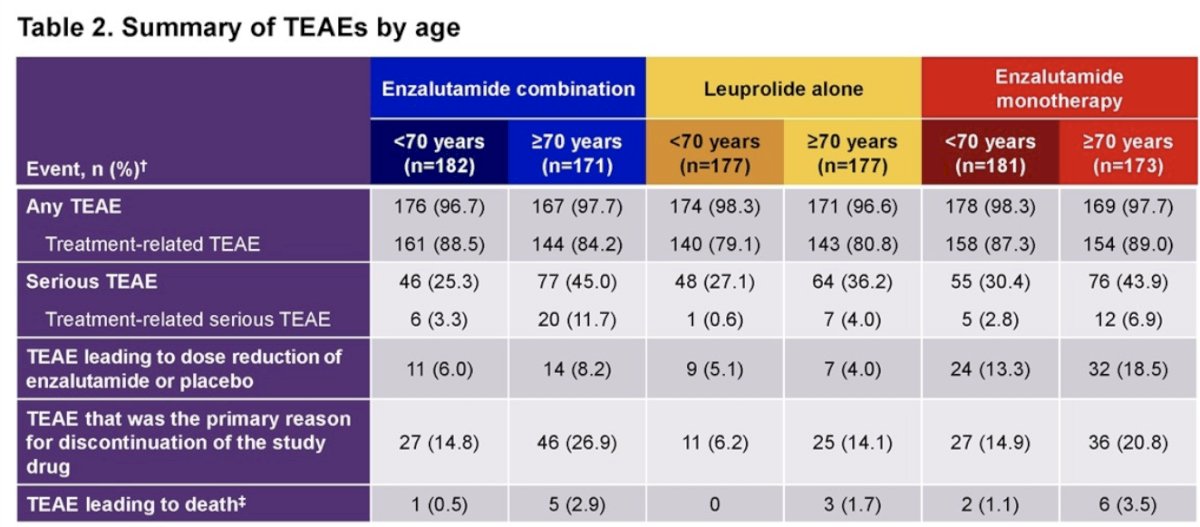
For clustered treatment emergent adverse events of special interest of any grade, musculoskeletal events, fatigue, and hypertension were common across all age and treatment groups.
Dr. Shore concluded his presentation by discussing a post hoc analysis from EMBARK focusing on age with the following take-home points:
- In patients with high-risk BCR, clinically meaningful benefits in metastasis free survival were observed with enzalutamide combination and enzalutamide monotherapy, regardless of age
- The observed treatment effect on metastasis free survival in treatment group comparisons was not statistically significantly different between age groups
- Treatment-related serious adverse events were more common in older patients but were low (0.6-11.7%) regardless of age
Presented by: Neal D. Shore, MD, FACS, Carolina Urologic Research Center, Atlantic Urology Clinics, Myrtle Beach, SC
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
Related content: EMBARK Trial: Age-Stratified Analysis of Enzalutamide in High-Risk Biochemically Recurrent Prostate Cancer - Neal Shore
References:


