The PROfound study met the primary endpoint, showing that olaparib provides a statistically significant improvement in radiographic progression-free survival (rPFS) versus control (physician’s choice of enzalutamide or abiraterone) in patients with mCRPC and HRR gene alterations in Cohort A (BRCA/ATM alterations).3,4 The study also met the prespecified secondary endpoint with a statistically significant improvement in overall survival (OS) compared with control in Cohort A (hazard ratio [HR] 0.69; 95% confidence interval [CI] 0.50, 0.97).4 For Cohort A+B and BRCAm populations, OS HRs for olaparib compared with control were 0.79 (0.61, 1.03)4 and 0.63 (0.42, 0.95),2 respectively.
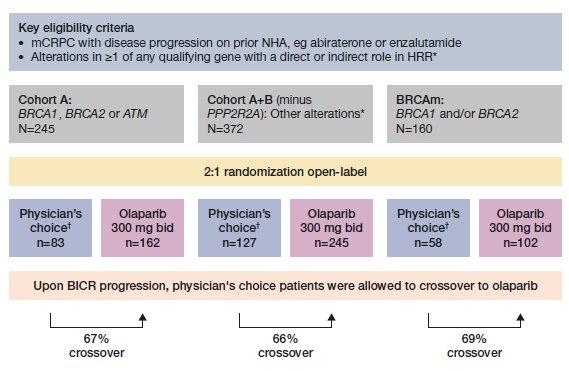
Figure 1. Rates of crossover in the PROfound study in Cohort A, A+B, and BRCAm populations. *BRCA1, BRCA2, ATM, BARD1, BRIP1, CDK12, CHEK1, CHEK2, FANCL, PALB2, PPP2R2A, RAD51B, RAD51C, RAD51D, RAD54L; †Either enzalutamide (160 mg qd) or abiraterone (1000 mg qd plus prednisone [5 mg bid]). BICR, blinded independent central review; mCRPC, metastatic castration-resistant prostate cancer; NHA, new hormonal agent
As permitted by the study protocol, 67%, 66% and 69% of patients in the control arm switched to olaparib following disease progression in Cohort A, Cohort A+B, and in the BRCAm cohort, respectively (Figure 1). Considering this large proportion of treatment switching, the OS results of PROfound may underestimate the survival benefit of olaparib treatment (Figure 2). This study aimed to comprehensively evaluate the impact of treatment switching on OS using the final data, comparing published treatment switching adjustment methods, and identifying the most appropriate method.
Treatment switching analyses were performed for the primary population (Cohort A) and the populations approved for treatment with olaparib by the FDA and EMA: Cohort A+B (excluding the PPP2R2A gene) and BRCAm. Five methods were explored to adjust for switching: excluding patients in the control arm who received subsequent olaparib, censoring patients in the control arm at the time of receiving subsequent olaparib, Rank Preserving Structural Failure Time Model (RPSFTM), Inverse Probability of Censoring Weights, and Two-Stage Estimation.
All adjustment methods evaluated demonstrated that the survival benefit for olaparib is likely to be greater than that observed in PROfound if switching from control to olaparib is adjusted for. This was consistent across all populations assessed, with the most considerable improvement in OS observed in the BRCAm subgroup.5
The RPSFTM was considered the most appropriate approach as the results were robust to sensitivity analysis testing of the statistical assumptions associated with this method. For Cohort A, the adjusted OS HR was between 0.42 and 0.52 for olaparib vs. control, depending on the choice of model selected for the RPSFTM selected (Figure 2). Median OS was between 11.7 and 12.6 months for control.
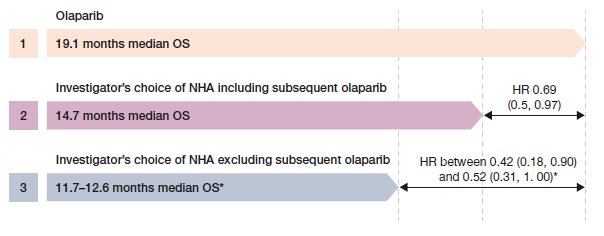
Figure 2. Overall survival hazard ratios for Cohort A adjusted for treatment switching using RPSFTM. *Depending on the RPSFTM selected. HR, hazard ratio for olaparib vs investigator’s choice of NHA; OS overall survival
These results demonstrate that the observed survival benefit of olaparib versus control in the intention-to-treat population of PROfound is likely to be underestimated. Overall, our findings reinforce the clinical benefit of olaparib in the treatment of HRRm mCRPC following disease progression on prior NHA.
Written by: Rachel Evans1 Neil Hawkins1 Pascale Dequen-O’Byrne1 Charles McCrea2 Dominic Muston3 Christopher Gresty2 Sameer R. Ghate3 Lin Fan3 Robert Hettle2 Keith R. Abrams1 Johann de Bono4 Maha Hussain5 Neeraj Agarwal6
- Visible Analytics, Oxford, UK
- AstraZeneca, Cambridge, UK
- Merck & Co., Inc., Kenilworth, NJ, USA
- Institute of Cancer Research and Royal Marsden, London, UK
- The Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Chicago, IL
- Oncology/Internal Medicine, Huntsman Cancer Institute, University of Utah, Salt Lake City, UT
References:
- US Food and Drug Administration (FDA). Highlights of prescribing information: LYNPARZA® (olaparib) tablets, for oral use. 2020. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208558s014lbl.pdf (accessed 23 March 2021).
- European Medicines Agency (EMA). Lynparza. 2018. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/lynparza (accessed 23 March 2021).
- de Bono J, Mateo J, Fizazi K et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med 2020;382:2091-102.
- Hussain M, Mateo J, Fizazi K et al. Survival with olaparib in metastatic castration-resistant prostate cancer. N Engl J Med 2020;383:2345-57.
- De Bono JS, Matsubara N, Penel N et al. Exploratory gene-by-gene analysis of olaparib in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC): PROfound. J Clin Oncol 2020;39:126.
Read the Abstract


