The phase III PROfound study (NCT02987543) showed a significant radiographic progression-free survival (rPFS) benefit in patients with metastatic castration-resistant prostate cancer (mCRPC) taking olaparib versus a control group receiving enzalutamide or abiraterone. All patients also had at least one alteration in the homologous recombination repair (HRR)-associated genes of BRCA1, BRCA2, or ATM.
In PROfound, safety was assessed in the total patient population, which included all patients who had at least one alteration in a predetermined set of 15 HRR-associated genes.
A predictable safety profile for patients receiving olaparib was reported and the most commonly occurring adverse events (AEs) of anaemia, nausea, fatigue/asthenia, and decreased appetite were generally manageable through dose modifications and supportive therapies without the need for treatment discontinuation.1
This is an important safety finding because to provide maximum benefit to the patient, new treatments need to be tolerable enough to enable patients to remain on treatment for as long as they are obtaining clinical benefit. A well-documented long-term safety profile is also a consideration for determining when treatment should begin i.e. the stage of patient’s disease. This is particularly relevant for diseases such as mCRPC that are challenging to treat, where patients face many treatment options that have different and sometimes cumulative toxicities. Current standard treatments for mCRPC are typically androgen deprivation therapy (ADT) combined with next generation hormone agents (NHA; such as abiraterone and enzalutamide) or chemotherapy, but new treatments like poly (ADP-ribose) polymerase (PARP) inhibitors (olaparib and rucaparib) and radioisotopes (lutetium and radium) have been approved to improve outcomes for patients with mCRPC.
Olaparib, a targeted cancer drug, is one of the approved PARP inhibitors
Olaparib belongs to an orally-administered class of drugs called PARP inhibitors that target HRR gene alteration. HRR is one of the repair pathways used by mammalian cells to correct DNA damage that may have occurred during cell division. Patients whose cancers have HRR gene alteration (the most commonly known are BRCA1 and BRCA2) can respond to olaparib because in the presence of a PARP inhibitor, the alteration prevents the tumour cell from correctly repairing the damaged DNA, ultimately leading to cell death. Hence, it is in these patients harbouring HRR alterations that PARP inhibitors are most effective.2
It is very helpful to clinicians treating patients with prostate cancer that olaparib already has a well-documented safety profile, having been first approved in 2014 for ovarian cancer and then in breast and pancreatic cancers. Approval for prostate cancer was granted in the USA in May 2020 for patients with germline or somatic HRR-altered mCRPC who had progressed on prior treatment with enzalutamide or abiraterone.3 In the European Union, approval for patients with mCRPC and BRCA1 and/or BRCA2 (BRCA) gene alterations who progressed on prior treatment with enzalutamide or abiraterone was granted in November 2020.4 The PROfound trial confirmed that the tolerability profile of olaparib in patients with mCRPC was similar to that seen in other solid tumour types.
As a result of the PROfound trial, monotherapy olaparib is approved and available for use in patients with mCRPC and qualifying HRR alterations and associated AEs are comparatively manageable. Approved companion diagnostic testing has also been sufficiently developed to enable the identification of patients in whom the drug is likely to be effective.
What AEs can patients expect when taking olaparib?
Of the four most commonly occurring AEs associated with olaparib (anaemia, nausea, fatigue/asthenia, and decreased appetite), we can expect these events to occur early (Figure 1), reach peak frequency within 2 months and then decline to a baseline level (Figure 2). These AEs can be managed by dose modifications and supportive therapies; discontinuation rates would be expected to be low and the majority of patients would be expected to remain on the full recommended dose. Anaemia is likely to be the most commonly occurring event as it is a class effect of PARP inhibitor treatment recognized from clinical trials of all PARP inhibitors (including olaparib, rucaparib, niraparib, talazoparib, and veliparib) in patients with solid tumours.5 Following the initial peak in frequency, anaemia can be expected to still occur throughout the treatment period, and a little over a third of events will be at or above Grade 3 by Common Terminology Criteria for Adverse Events version 4.0 grading, i.e. may require supportive intervention or dose modifications, therefore, frequent clinical monitoring is advised in accordance with the prescribing information. Supportive treatments include blood transfusion (red blood cell products and whole blood transfusions) and erythropoiesis-stimulating agents. Additionally, given the potential effect of radiation therapy for bone metastases on anaemia, study treatment was interrupted for patients in PROfound for a minimum of 3 days before palliative radiation treatment, and could only be resumed within 4 weeks as long as any bone marrow toxicity had recovered. Not every patient with anaemia will need supportive therapy; in PROfound, 127 (50%) patients receiving olaparib experienced an AE of anaemia (grouped term), which for 58 (23%) patients was at or above Grade 3. In total, 71 of the 127 (56%) patients had a blood transfusion and/or another anti anaemic preparation as a concomitant medication, and 42/127 (33%) patients had a dose reduction due to anaemia. There did not appear to be an increased risk of anaemia with olaparib treatment in patients with more bone metastases or who received prior taxanes.
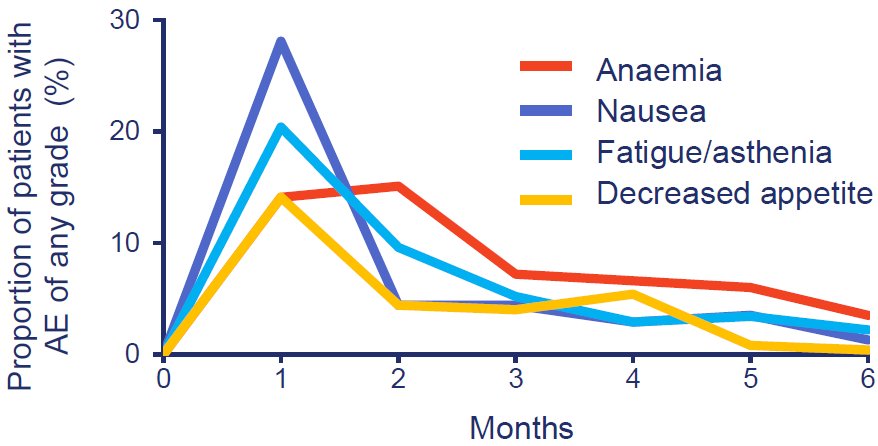
Figure 1: The most common adverse events in patients receiving olaparib peaked within the first 2 months and were mostly Grade 1 or 2. The median total duration of treatment for patients receiving olaparib was 7.6 months (range 0.03–28.9).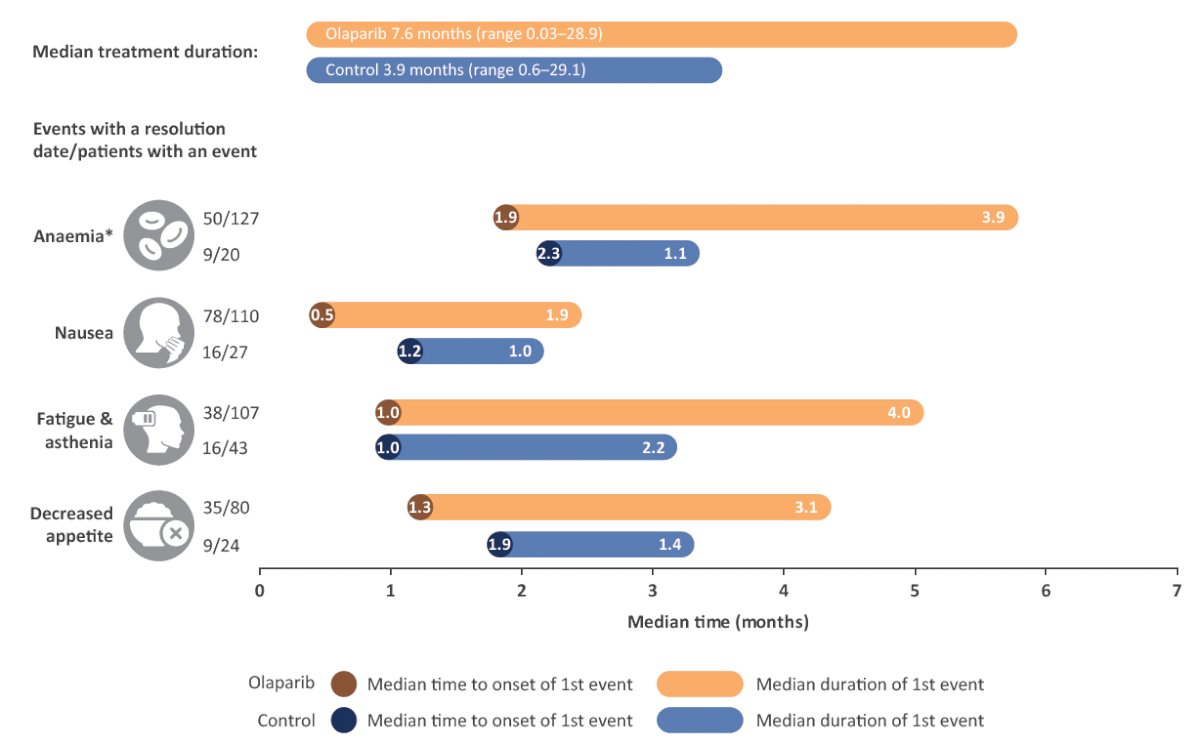
Figure 2: Median time to first onset and duration of the most common AEs (all causality) in patients who experienced the event.
These AEs are expected to have been actively managed where appropriate during treatment.
*Anaemia is reported as a ‘grouped term’ based on Medical Dictionary for Regulatory Activities (MedDRA)-preferred terms and includes anaemia, decreased haemoglobin level, decreased red blood cell count, decreased haematocrit level, erythropenia, macrocytic anaemia, normochromic anaemia, normochromic normocytic anaemia and normocytic anaemia.
Less common and potentially more serious AEs observed during olaparib’s clinical trial programme include pneumonitis, myelodysplastic syndrome/acute myeloid leukemia (MDS/AML), and thromboembolic disorders. In PROfound, pneumonitis occurred infrequently in 2% and 1.5% of patients in the olaparib and control group of patients receiving enzalutamide or abiraterone, respectively. Similarly, MDS/AML was observed in only one patient (0.4%) in the olaparib group after a 30-day follow-up period. If pneumonitis or MDS/AML events occur, olaparib treatment should be interrupted when an event is suspected and discontinued if confirmed, as per the prescribing information.3,4 Thromboembolic disorders are an inherent risk in patients with mCRPC through their association with the use of continuous ADT, retroperitoneal lymphadenopathy in some patients, and reduced exercise and mobility.6,7 Venous thromboembolic events, including pulmonary embolism, may also be associated with the use of olaparib. Venous thromboembolic events are seen more often with olaparib across tumour types but more prominently in the prostate indications. The mechanism of this association is unknown. In PROfound, venous thromboembolic events occurred in 8% of olaparib and 3% of control patients. Three patients had treatment interruptions due to these events (two in the olaparib and one in the control group) and one patient in the olaparib group discontinued treatment. The most common thromboembolic event, pulmonary embolism, was observed in 12 (5%) patients in the olaparib group and 1 (1%) patient in the control group. Time to onset in the olaparib group ranged from 0.2 to 11.3 months. The majority of patients with pulmonary embolism in PROfound were asymptomatic and identified incidentally with computerized tomography scans of the chest. Typically, patients recovered from venous thromboembolic events and were able to continue with olaparib treatment following the introduction of anticoagulant treatment based on standard medical practice.1 Pulmonary embolisms have been observed in other PARP inhibitor trials in patients with mCRPC. For example, in the phase II trial of talazoparib, 6% of patients treated with talazoparib monotherapy had a pulmonary embolism episode.8 In consideration of the clinical experience with prostate cancer, it remains important to consider thromboembolic side effects when treating patients with mCRPC and to treat as medically appropriate, which may include long-term anticoagulation as clinically indicated by the US prescribing information.3
What does the future hold for PARP inhibitors?
The clinical development of PARP inhibitors continues apace for the treatment of mCRPC. Based on the results of the phase II TRITON 2 trial, rucaparib, like olaparib, is also approved in the USA for the treatment of BRCA-altered mCRPC.9 The confirmatory phase III TRITON3 trial recently met its primary endpoint of a significant improvement in rPFS versus the physician’s choice of docetaxel or second-generation androgen pathway inhibitor in the BRCA-altered subgroup.10 Approval for rucaparib was granted in the same month and year as olaparib (May 2020), although their labels differ regarding prior therapies. For rucaparib, patients need to have been previously treated with androgen receptor-directed therapy and a taxane-based chemotherapy, whereas for olaparib, although docetaxel was allowed, prior treatment with enzalutamide or abiraterone is mandatory. Other PARP inhibitors, such as talazoparib, niraparib and fluzoparib are also in development as monotherapies or in combination with standard treatments.
With regard to combination therapies, the PROpel trial, which evaluated the combined effect of a PARP inhibitor (olaparib) with standard treatment (abiraterone) as first-line treatment in patients with mCRPC unselected by HRR alteration status, reported a positive outcome in such a patient population. At first data cut-off, PROpel met its primary endpoint of a statistically significant improvement in rPFS with olaparib plus abiraterone versus abiraterone alone (24.8 vs 16.6 months; hazard ratio (HR), 0.66; 95% confidence interval [CI] 0.54–0.81; P<0.001) and with safety profiles consistent for those of the individual drugs.11 rPFS benefit was more pronounced for patients with BRCA alterations than those without. Adverse events of note in the olaparib plus abiraterone arm included anaemia (15.1% of patients at Grade ≥3) and venous thromboembolic events (7.3% of patients, all grades).12 In December 2022, the European Commission approved olaparib in combination with abiraterone and prednisone or prednisolone for the treatment of patients with mCRPC in whom chemotherapy is not clinically indicated.13 Two further PARP inhibitor combination trials in patients with mCRPC are of note. Firstly, in the MAGNITUDE trial, niraparib combined with abiraterone as a first-line therapy for mCRPC reported a positive outcome for patients with HRR gene alterations, with the greatest benefit in patients with BRCA alterations.14 Initial results showed the combination statistically improved rPFS in a cohort of patients with BRCA1/2 alterations compared with abiraterone alone (16.6 vs 10.9 months; HR, 0.53; 95% CI 0.36–0.79; P=0.0014). There was no evidence of clinical benefit in patients without HRR gene alterations.14 The second trial of interest is TALAPRO-2, in which talazoparib is combined with enzalutamide as a first-line mCRPC therapy. The study met its primary endpoint showing a statistically significant improvement in rPFS compared with enzalutamide alone (median not reached vs 21.9 months; HR, 0.63; 95% CI 0.51–0.78; P<0.001).15 Notable adverse events in the talazoparib plus enzalutamide arm included anaemia (46.5% of patients at Grade ≥3) and gastrointestinal toxicity.15
PARP inhibitor plus NHA combinations have shown the greatest benefit in patients with BRCA alterations and should probably become standard of care in this population. Treatment benefits in patients without a BRCA alteration are more modest, however, results from the phase III PROpel and TALAPRO-2 trials suggest a PARP inhibitor plus NHA combination has confirmed anticancer activity in this population. Analyses of longer-term data to further understand the benefit/risk ratio will be key to assessing these combinations' exact role in the non-BRCA altered populations.
In addition to approvals in the mCRPC setting, several studies are ongoing to evaluate PARP inhibitors in the metastatic hormone-sensitive prostate cancer (mHSPC) setting. The ongoing phase II PROact study (NCT05167175) is evaluating olaparib plus abiraterone in patients with mHSPC and HRR alterations, while a phase II study (NCT04734730) is evaluating talazoparib added to first-line androgen deprivation therapy and abiraterone in mHSPC. The phase III TALAPRO-3 study (NCT04821622) evaluates talazoparib plus enzalutamide versus placebo plus enzalutamide in patients with mHSPC and HRR alterations. Furthermore, the ongoing phase III AMPLITUDE study (NCT04497844) is evaluating niraparib plus abiraterone in patients with mHSPC and HRR alterations, and the phase II TRIUMPH study (NCT03413995) is evaluating rucaparib monotherapy in patients with mHSPC and germline HRR alterations. In addition, AZD5305, a PARP1-selective inhibitor, is being evaluated in combination with physician’s choice of NHA in the phase I/IIa PETRANHA study in patients with mHSPC and mCRPC (NCT05367440).
It is likely that not all PARP inhibitors are the same with regards to tolerability16 and clinicians may one day be faced with the conundrum of which PARP inhibitor to prescribe, in which combination and at what stage of disease (mHSPC or mCRPC). To help make their decision, the safety profiles of all PARP inhibitors, whether administered alone or in combination, will shortly be subject to even greater scrutiny. Time will tell if the next step forward for PARP inhibitors lies in their combination with hormonal therapies administered at an earlier disease stage.
Written by:- Guilhem Roubaud, Department of Medical Oncology, Institut Bergonié, Bordeaux, France
- Mustafa Özgüroğlu, Department of Internal Medicine, Division of Medical Oncology, Cerrahpasa Faculty of Medicine, Istanbul University-Cerrahpasa, Istanbul, Turkey
- Craig Gedye, Calvary Mater Newcastle, Waratah, Australia
- Niven Mehra, Radboud University Medical Center, Nijmegen, the Netherlands
- Karim Fizazi, Department of Cancer Medicine, Institut Gustave Roussy, University of Paris Saclay, Villejuif, France
The PROfound study was sponsored by AstraZeneca and is part of an alliance between AstraZeneca and Merck Sharp & Dohme LLC, a subsidiary of Merck & Co. Inc., Rahway, NJ, USA. Medical writing assistance was provided by Martin Goulding, DPhil, from Mudskipper Business Ltd, funded by AstraZeneca and Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
References:- de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med 2020;382(22):2091-2102. DOI: 10.1056/NEJMoa1911440.
- Antonarakis ES, Gomella LG, Petrylak DP. When and How to Use PARP Inhibitors in Prostate Cancer: A Systematic Review of the Literature with an Update on On-Going Trials. Eur Urol Oncol 2020;3(5):594-611.
- FDA. LYNPARZA® (olaparib) Prescribing Information. AstraZeneca, Gaithersburg, MD. May
- European Medicines Agency (EMA). LYNPARZA (olaparib). Summary of product characteristics.
- Zhou JX, Feng LJ, Zhang X. Risk of severe hematologic toxicities in cancer patients treated with PARP inhibitors: a meta-analysis of randomized controlled trials. Drug Des Devel Ther 2017;11:3009-3017.
- Abdol Razak NB, Jones G, Bhandari M, Berndt MC, Metharom P. Cancer-Associated Thrombosis: An Overview of Mechanisms, Risk Factors, and Treatment. Cancers (Basel) 2018;10(10).
- Zhan P, Wang Q, Qian Q, Yu LK. Risk of venous thromboembolism with the erythropoiesis-stimulating agents (ESAs) for the treatment of cancer-associated anemia: a meta-analysis of randomized control trials. Chin Clin Oncol 2012;1(2):19.
- de Bono JS, Mehra N, Scagliotti GV, et al. Talazoparib monotherapy in metastatic castration-resistant prostate cancer with DNA repair alterations (TALAPRO-1): an open-label, phase 2 trial. Lancet Oncol 2021;22(9):1250-1264.
- FDA. RUBRACA (rucaparib) prescribing information.
- .Fizazi K, Piulats JM, Reaume MN, et al. Rucaparib or Physician’s Choice in Metastatic Prostate Cancer. New England Journal of Medicine 2023;388(8):719-732.
- Clarke NW, Armstrong AJ, Thiery-Vuillemin A, et al. Abiraterone and Olaparib for Metastatic Castration-Resistant Prostate Cancer. NEJM Evidence 2022;1(9):EVIDoa2200043.
- Clarke NW, Armstrong AJ, Thiery-Vuillemin A, et al. Final overall survival (OS) in PROpel: Abiraterone (abi) and olaparib (ola) versus abiraterone and placebo (pbo) as first-line (1L) therapy for metastatic castration-resistant prostate cancer (mCRPC). Journal of Clinical Oncology 2023;41(6_suppl):LBA16-LBA16.
- European Commission. Union Register of medicinal products for human use. Product information for Lynparza.
- Chi KN, Rathkopf DE, Smith MR, et al. Phase 3 MAGNITUDE study: First results of niraparib (NIRA) with abiraterone acetate and prednisone (AAP) as first-line therapy in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) with and without homologous recombination repair (HRR) gene alterations. Journal of Clinical Oncology 2022;40(6_suppl):12.
- Agarwal N, Azad A, Carles J, et al. TALAPRO-2: Phase 3 study of talazoparib (TALA) + enzalutamide (ENZA) versus placebo (PBO) + ENZA as first-line (1L) treatment in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). Journal of Clinical Oncology 2023;41(6_suppl):LBA17-LBA17.
- Bao S, Yue Y, Hua Y, et al. Safety profile of poly (ADP-ribose) polymerase (PARP) inhibitors in cancer: a network meta-analysis of randomized controlled trials. Ann Transl Med 2021;9(15):1229. DOI: 10.21037/atm-21-1883.


