(UroToday.com) The 2022 ASCO annual meeting featured a session on prostate cancer, including a presentation by Dr. Fred Saad discussing the association of PSA response and overall survival in patients with mHSPC from the phase 3 ARASENS trial. Reductions in PSA level have been associated with improved OS in patients with mHSPC. In mHSPC, PSA response has been identified as a relevant biomarker, and undetectable PSA levels (0.2 ng/dL) have been associated with better clinical outcomes. Darolutamide is a highly potent and structurally distinct androgen receptor inhibitor that demonstrates low blood-brain barrier penetration and has a low potential for drug-drug interactions. In ARASENS,1 darolutamide + ADT in combination with docetaxel significantly reduced the risk of death by 32.5% (HR 0.675, 95% CI 0.568–0.801; p < 0.0001) vs ADT + docetaxel in patients with mHSPC. At ASCO 2022, Dr. Saad and colleagues report the association between PSA response and OS from ARASENS.
Patients with mHSPC were randomized 1:1 to darolutamide 600 mg twice daily or matching placebo + ADT and docetaxel. Serum PSA was measured at screening and every 12 weeks. Exploratory analyses included time to PSA progression (≥25% increase from PSA nadir [lowest or at study entry] and PSA increase ≥2 ng/mL ≥12 weeks from nadir [both confirmed by a second value ≥3 weeks later]) and undetectable PSA (< 0.2 ng/mL for 2 samples ≥3 weeks apart) at 24, 36, and 52 weeks and any time during treatment. Comparisons between treatment groups were performed using the Cochran-Mantel Haenszel test stratified by randomization stratification factors (metastatic spread according to TNM classification and alkaline phosphatase levels at study entry). Post hoc landmark analyses evaluated the association between undetectable PSA at weeks 24 and 36 and OS for the overall population.
Among 1,306 randomized patients, 1305 were included in the full analysis set (darolutamide 651; placebo 654), both with ADT and docetaxel. Median PSA levels at study entry were 30.3 (range 0.0–9219.0) and 24.2 (range 0.0–11,947.0) ng/mL, respectively. Darolutamide significantly prolonged time to PSA progression (HR 0.26, 95% CI 0.21–0.31; p < 0.0001):
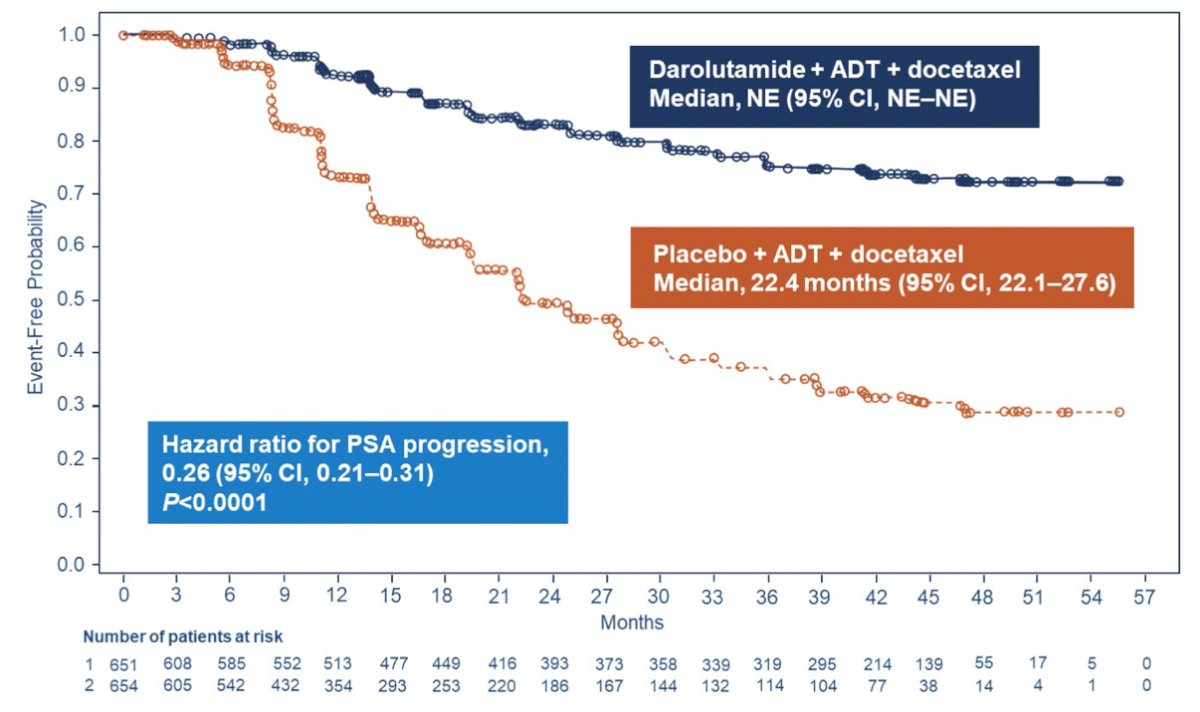
Undetectable PSA was achieved in more patients receiving darolutamide (48.7%) vs placebo (23.9%) at 24 weeks, and the rate continued to increase at 36 and 52 weeks in the darolutamide group to 57.1% and 60.2%, respectively, vs minimal change in the placebo group (25.1% and 26.1%). Undetectable PSA levels at any time were achieved in 67.3% in the darolutamide group and 28.6% in the placebo group. A treatment difference in undetectable PSA based on non-overlapping 95% CIs was observed at all time points:

For the overall population, OS was improved for patients who achieved undetectable PSA levels vs those who did not at 24 weeks (HR 0.47, 95% CI 0.35–0.63) and 36 weeks (HR 0.37, 95% CI 0.28–0.49):

Dr. Saad concluded this presentation by discussing the association between PSA response and overall survival in patients with mHSPC from the phase 3 ARASENS trial with the following take-home messages:
- The combination of darolutamide + ADT and docetaxel significantly prolonged the time to PSA progression and more patients receiving darolutamide vs placebo achieved undetectable PSA levels, reflecting strong PSA response over time
- In patients with mHSPC, achievement of undetectable PSA at 24 and 36 weeks was associated with improved OS, with risk of death reduced by 53% and 63%, respectively, compared to those who did not achieve undetectable PSA at 24 and 36 weeks
Presented by: Fred Saad, MD, FRCS, University of Montréal Health Center, Montréal, QC, Canada
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 3 – Mon, June 7, 2022.
References:


