(UroToday.com) At the Annual Meeting of the Society of Urologic Oncology there was a session focused on prostate specific membrane antigen (PSMA) and prostate cancer. In the context of this session, Dr. Scott Tagawa presented on the evolving role of prostate cancer theranostics, including not just the VISION trial but subsequent data.
Dr. Tagawa began by noting that the idea of targeted radionuclide therapy is not a novel idea. This principle, pairing imaging with 123-Iodine with therapeutic 131-Iodine, has been utilized in thyroid cancer for years. He further noted that bone targeting therapy such as strontium-89, samarium-153, and radium-223 are in theory driven by imaging results from a standard technetium bone scan. However, in prostate cancer, the true pairing of imaging and therapeutic interventions emerged with the advent of PSMA imaging with Lu177 therapeutics.
For context, he noted that PSMA is “the single most well-established, prostate-restricted, cell membrane target known”. This has been validated in both cellular lines and in in vivo data. The principle that PSMA is prostate restricted is, however, he noted note entirely true – there is luminal expression in many other cancers, in the proximal renal tubules, in the brush border of the small intestine, and in salivary and lacrimal glands. Dr. Tagawa noted that attenuated androgen receptor (AR) signalling is a hallmark of lethal prostate cancer. It has been recognized from both in vitro and in vivo studies that AR signalling pathway activity is inversely related to PSMA expression. Despite nearly, though not completely, universal expression in prostate cancer, Dr. Tagawa noted that there is substantial heterogeneity in PSMA expression both within and between tumors.
Moving beyond the target, Dr. Tagawa noted that radiotherapy is a cornerstone in the treatment of prostate cancer. Traditional local approaches to radiotherapy delivery including external beam radiotherapy and brachytherapy may be administered as curative therapy. In the context palliative therapy, both local external beam radiotherapy and systemic radiotherapy using radium-223 (and more recently, 177-Lu-PSMA) provide benefit.
Given this context, Dr. Tagawa then defined PSMA-TRT (prostate-specific membrane antigen-targeted radionuclide therapy) as the administration of systemic radiotherapy, targeted to PSMA-avid cells. There are two key components to these molecules. First, there is a PSMA-targeting vehicle. There are a variety of these that have differing properties, predominately related to the size (small molecule vs antibody), that affect their kinetics and biodistribution. Second, there is a radionuclide, the radioactive particle. These have typically been beta- and alpha-emitters though there is increasing interest in auger for therapy and gamma for imaging. The efficacy and toxicity of these are partially related to their specific properties, in a manner that will be discussed further below.
Dr. Tagawa noted that there are a large number of PSMA-TRT agents that are either approved or in trials. These include a variety of different PSMA-targeting vehicles and therapeutic payloads.
In terms of clinical benefit, Dr. Tagawa noted that some of the early data on the use of PSMA-base theranostics has come from retrospective data from Germany. Most of these men had previously received conventional treatment approaches and were selected by therapy on the basis of avidity of PSMA-PET/CT. While promising data emerged from these studies, Dr. Tagawa noted the limitations in the completeness of both oncologic efficacy data and toxicity. TheraP represented some of the first prospective data on the efficacy of 177-Lu-PSMA. Subsequently, the VISION trial provided prospective, randomized data assessing this treatment approach. Based on data from each of these trials, Dr. Tagawa noted that the “prime time” for PSMA-TRT is now.

He then asked where we may go from here, moving forward. He noted that there are ongoing improvements in beta-radiolabelled products. First, there is ongoing work to optimize the dosing and treatment schedule of small molecules. Second, there are efforts to optimize patient selection. To this end, there is assessment of the role of PSMA-TRT across the spectrum of disease ranging from mCRPC following many lines of therapy where it is currently indicated to earlier in mCRPC space, and into non-castrate disease. Further there is work to identify radiographic, clinical, and genomic predictors of response and benefit. Third, there are a few reported and many ongoing trials assessing the combination of PSMA-TRT with other agents including androgen-receptor pathway targeting agents, chemotherapy, PARP inhibitors, and others. Beyond these improvements in the delivery of beta-radiolabelled products, there are substantial efforts to develop and assess alpha or auger-radiolabeled constructs.
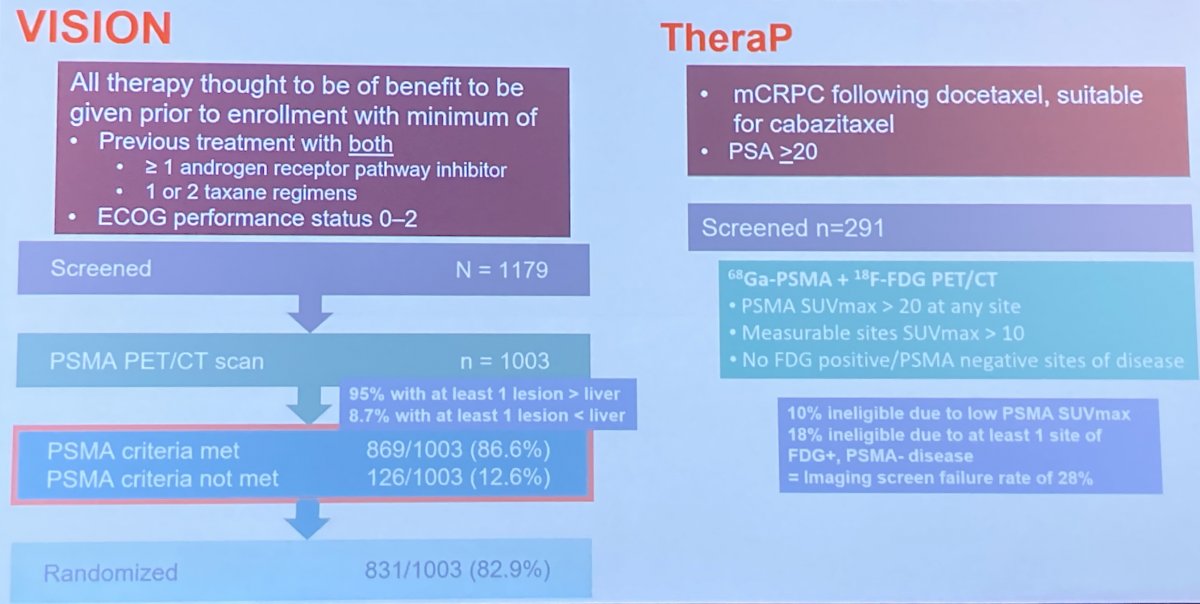
In terms of identifying patients for PSMA-TRT, Dr. Tagawa noted that the clinical indications and radiographic inclusion of the two trials to date differed. First, while those patients enrolled on VISION had to have received at least one androgen receptor pathway inhibitor and at least one taxane regime (as well as exhausting other treatment approaches such as radium-223 and PARP inhibitors), TheraP enrolled patients following docetaxel therapy (most of whom had also received an androgen receptor pathway inhibitor) who were eligible for cabazitaxel. Second, radiographic inclusion criteria differed. In VISION, screening required that patients have PSMA avidity in there disease sites above the level of the liver. This relatively lax criteria resulted in approximately 12% of patients being ineligible. In contrast, TheraP required both PSMA-PET and FDG-PET with a requirement for high PSMA avidity and no discordant lesions. Thus, TheraP had an imaging screen failure rate of 28%.
In the VISION trial, subgroup analyses assessing PSMA-PET characteristics that are prognostic for response among those receiving PSMA-TRT have identified whole body SUVmean as prognostic for both rPFS and OS. Interesting, in TheraP, these analyses have included patients in the control arm. Thus, the authors were able to show that PSMA-PET/CT parameters are not just prognostic, but also predictive.

Moving to the question of treatment approach, Dr. Tagawa highlighted important differences between alpha and beta-particles. Importantly, alpha particles have much higher energy per particle but travel a much shorter distance in tissue. In clinical practice, the alpha emitter 225-Ac has been utilized though the literature is essentially limited to case reports and pooled case series. These, however, suggest promising results. Phase I data suggest that 225Ac linked to the antibody J591 is tolerable with early evidence of clinical activity. However, there are multiple ongoing studies that will better define its role in the prostate cancer landscape. Additionally, there are numerous other PSMA-targeted alpha emitters being developed with various combinations of the PSMA-targeting vehicle and radionuclide payload.
Further considerations regarding treatment approach include the PSMA-targeting vehicle. There has been considerable work comparing antibodies versus minibodies versus small molecules.
Finally, Dr. Tagawa noted the considerable interest and many ongoing studies assessing combinations of PSMA-TRT with nearly every other treatment approach that has been utilized in prostate cancer, including chemotherapy, immunotherapy, novel hormonal therapies, PARP inhibitors, and triplets of these approaches. Combination therapy has potentially a large number of relevant considerations including alterations of the target (as AR-targeting therapies may change PSMA expression), radiosensitization, and effects of uptake or retention of small molecules/antibodies.
Before closing, he noted that PSMA is expressed in the neovasculature of most solid tumors. Thus, this theranostic approach may have utility outside the prostate cancer space. Further, use of antibody drug conjugates, bispecifics, and potential CAR-T may allow targeting of novel targets. Conceptually, theranostics has the benefit of allowing the combination of imaging based identification of disease followed by targeted treatment.
Thus, in concluding, he noted that PSMA is a clinically-validated, consistent cell surface target in prostate cancer that has real world importance. Both antibodies and small molecules allow targeting. Thus, we can exploit the selectivity of PSMA expression to deliver high doses of radiotherapy to tumors with relative sparing of normal tissue.
Presented by: Scott T. Tagawa, MD, MS, FACP, Professor of Medicine and Urology at Weill Cornell Medicine

