(UroToday.com) The 2023 ASCO annual meeting included a prostate cancer session, featuring a presentation by Dr. Yu Yang Soon discussing an exploratory analysis of the effects of 177Lu-PSMA-617 (LuPSMA) on overall survival in TheraP versus VISION randomized trials. LuPSMA in metastatic castration resistant prostate cancer was evaluated in the VISION and TheraP randomized trials and reported remarkably different effects on overall survival:
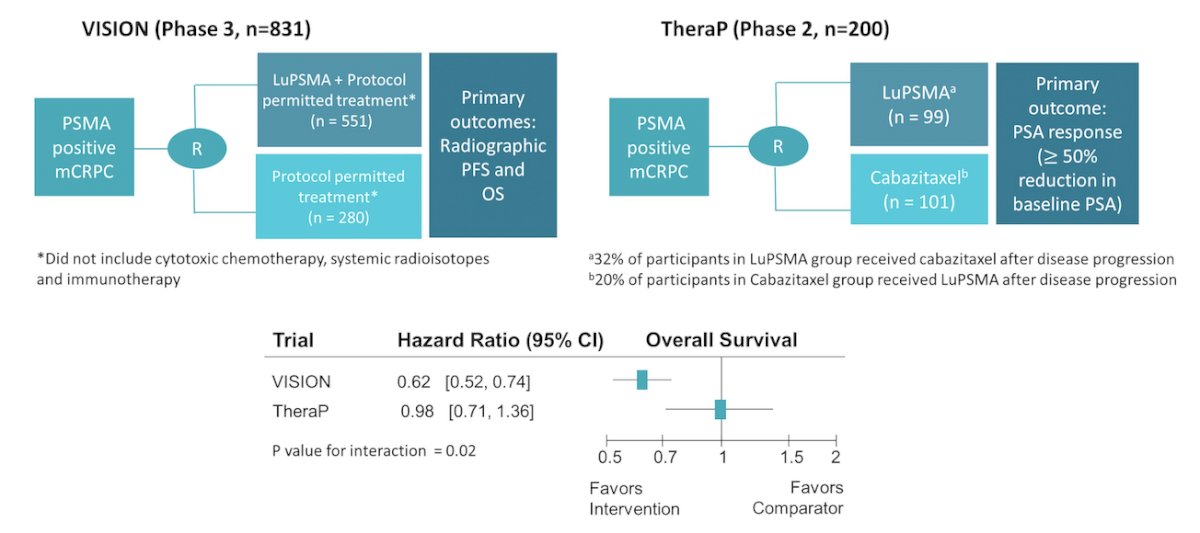
TheraP randomized participants to LuPSMA or cabazitaxel, in which the cabazitaxel group (n = 101) included 20 participants who crossed over to LuPSMA, and the LuPSMA group (n = 99) included 32 who crossed over to cabazitaxel.1 VISION randomized participants to treatment with or without LuPSMA, plus physicians’ choice among protocol-defined treatments, excluding cabazitaxel.2 Furthermore, 38% of control group participants in VISION had received cabazitaxel prior to randomization. This study investigated explanations for differences in these RCTs in the observed effects of LuPSMA on overall survival.
Dr. Soon and colleagues evaluated possible explanations for the differing hazard ratios for overall survival. This was done by comparing baseline characteristics from VISION and TheraP, exploring LuPSMA’s effect on overall survival if treatment switching on progression did not occur in TheraP. Switching-adjusted estimates were obtained using rank-preserving structure failure time model and inverse probability censoring weighting approaches. Individual time-to-event data from VISION was extracted from the published survival curves, and overall survival curves from the two trials were reconstructed using Kaplan-Meier methods and compared using the Cox proportional hazards model.
Baseline characteristics were similar except that prior cabazitaxel was used by 38% in VISION versus 0% in TheraP. Overall survival was longer in the comparator group of TheraP than the comparator group of VISION (HR 0.57, 95% CI 0.43 - 0.75) while OS was similar in the LuPSMA groups of VISION and TheraP (HR 0.92, 95% CI 0.70 - 1.19):
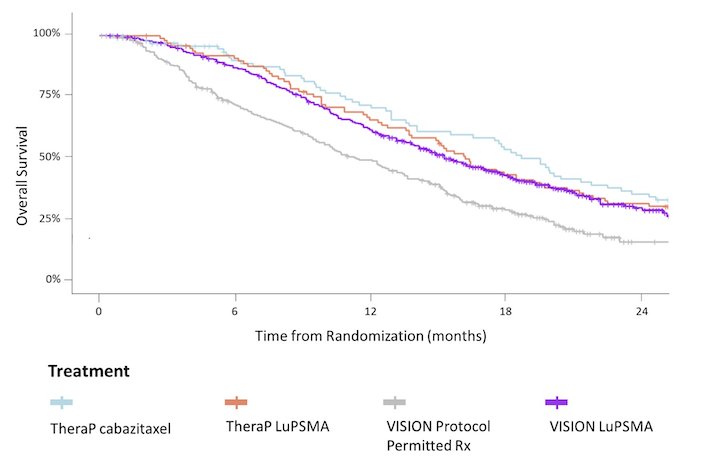
No significant differences in OS between the LuPSMA and cabazitaxel groups of TheraP were apparent using counterfactual analyses assuming crossovers had not occurred:
- No crossover from cabazitaxel: rank-preserving structural failure time HR 0.97, 95% CI 0.62 –1.52, inverse probability of censoring weighting HR 0.92, 95% CI 0.65-1.32
- No crossover from LuPSMA: rank-preserving structural failure time HR 0.97, 95% CI 0.60–1.58, inverse probability of censoring weighting HR 0.82, 95% CI 0.55–1.24
- No crossover from LuPSMA or cabazitaxel: rank-preserving structural failure time HR 0.97, 95% CI 0.53-1.74, inverse probability of censoring weighting HR 0.82, 95% CI 0.53–1.27
Dr. Soon concluded this presentation discussing an exploratory analysis of the effects of LuPSMA on overall survival in TheraP versus VISION randomized trials with the following take-home points:
- Hazard ratios for overall survival differed in VISION vs TheraP
- This difference was not explained by treatment switching on progression in TheraP
- Overall survival across trials was similar in the experimental groups treated with LuPSMA, but different in the control groups
- Thus, the choice of comparator matters
Presented by: Yu Yang Soon, NHMRC Clinical Trials Centre, The University of Sydney, Sydney, Australia
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.
References:
- Hofman MS, Emmett L, Sandhu S, et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomized, open-label, phase 2 trial. Lancet. 2021 Feb 27;397(10276):797-804.
- Sartor O, de Bono J, Chi KN et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021 Sep 16;385(12):1091-1103.


