(UroToday.com) The 2023 ASCO annual meeting included a prostate cancer session, featuring a presentation by Dr. Bernd Krause discussing a VISION trial sub-study analysis assessing tumor dosimetry of 177Lu-PSMA-617 for the treatment of metastatic castration-resistant prostate cancer (mCRPC). Despite significant therapeutic advances in recent years, mCRPC remains an incurable and fatal disease. In the landmark VISION trial, 177Lu-PSMA-617 + protocol-permitted standard of care significantly improved overall survival and radiographic progression-free survival compared with standard of care alone, in patients with PSMA-positive mCRPC.1 Additionally, in a VISION dosimetry sub-study, 177Lu-PSMA-617 had a good safety profile with low radiotoxicity in at-risk organs. In fact, dosimetry studies are a legal requirement for nuclear medicine therapies in certain countries. At the ASCO 2023 annual meeting, Dr. Krause and colleagues estimated the dosimetry of tumors after the administration of 177Lu-PSMA-617.
The VISION sub-study was performed in a separate cohort of 30 non-randomized participants at four sites in Germany, and dosimetry was performed in 29 patients. Eligible patients received 177Lu-PSMA-617 (7.4 GBq every 6 weeks, ≤ 6 cycles) + standard of care. Patients then underwent single-photon emission computed tomography/computed tomography scans at ~2, 24, 48 and 168 hours after the first administration of 177Lu-PSMA-617 (Cycle 1). Thereafter, up to five tumors in each patient were selected according to tumor size and relative activity uptake. Tumor delineation, volume, and morphology were determined using PET/CT images, and kinetic (time-activity) data were determined using SPECT images and the application of phantom derived recovery coefficients. Tumor dosimetry was estimated using the standard Medical Internal Radiation Dose/Radiation Dose Assessment Resource (MIRD/RADAR) method for internal dosimetry, and S-values were determined using CT-derived tumor volumes and tissue type as input to the Internal Dose Assessed by Computer (IDAC-Dose) 2.1 program. Finally, tumor dosimetry estimates were reported as absorbed dose per unit activity (Gy/GBq) and cumulative estimated absorbed dose (Gy) over all 6 cycles (44.4 GBq cumulative activity).
There were 104 tumors analyzed after the administration of 177Lu-PSMA-617 in Cycle 1. Tumor sites included bone (n = 84), lymphatic tissue (n = 18), lung tissue (n = 1), and soft tissue (n = 1). The mean tumor mass by site was 14.6 (SD 29.8; range 0.49–224) grams for bone and 12.5 (SD 15.9; range 0.68–60.7) grams for lymphatic tissue. The mean radiation-absorbed dose for all tumors was 6.5 (SD 8.4; range 0.13–55) Gy/GBq, and the median absorbed dose was 4.4 Gy/GBq. A summary of the tumor masses is as follows:
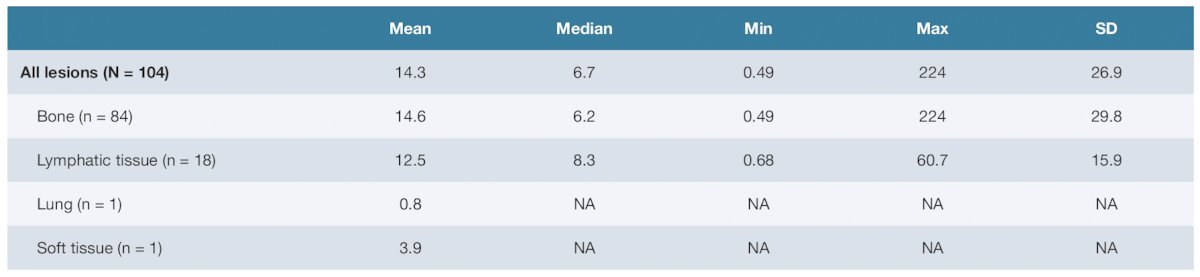
The mean radiation-absorbed dose by tumor site was 5.4 (SD 6.0; range 0.13–45) Gy/GBq for bone and 9.7 (SD 12; range 0.99–55) Gy/GBq for lymphatic tissue:
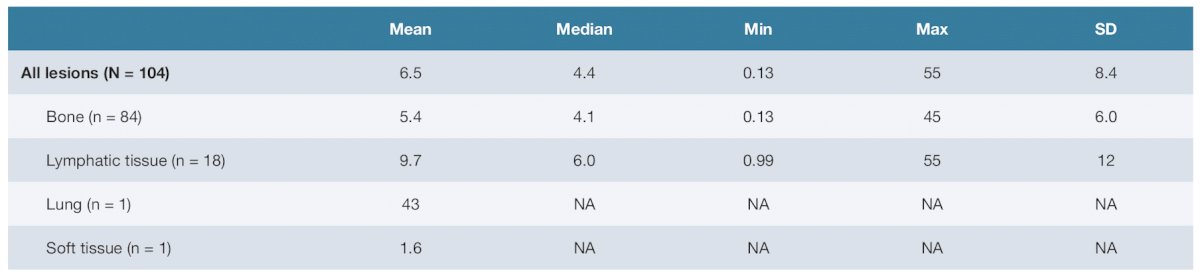
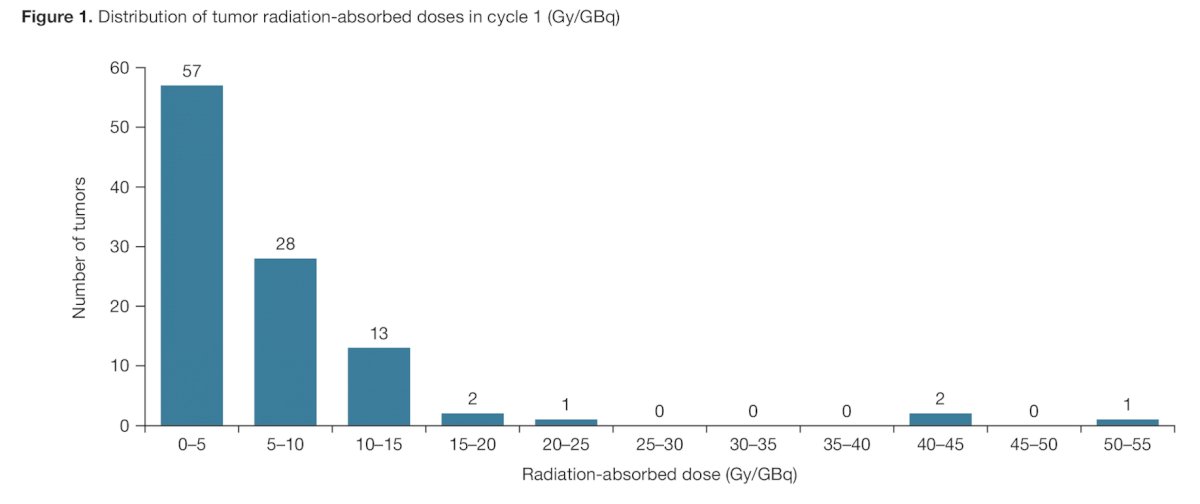
The distribution of tumor radiation-absorbed doses for all tumors is shown as follows:
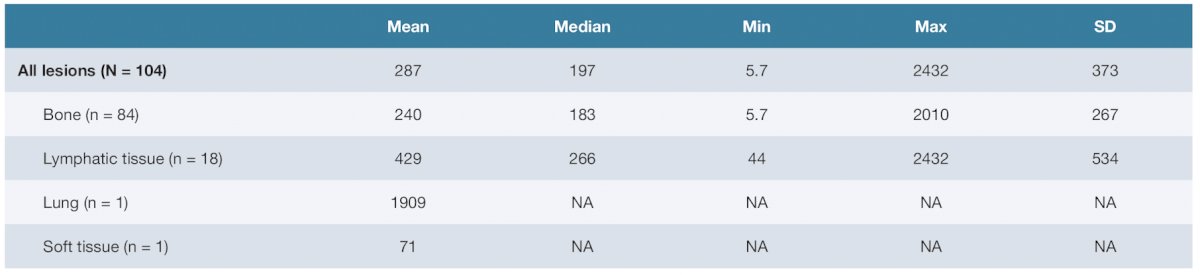
Finally, the 6-cycle cumulative estimated absorbed dose for all tumors was 287 (SD 373; range 5.7–2432) Gy. The 6-cycle cumulative estimated absorbed dose by tumor site was 240 (SD 267; range 5.7–2010) Gy for bone and 429 (SD 549; range 44–2432) Gy for lymphatic tissue:
Dr. Krause concluded this presentation by discussing a VISION trial sub-study analysis assessing tumor dosimetry of 177Lu-PSMA-617 for the treatment of mCRPC with the following take-home points:
- Tumor dosimetry estimates after administration of 177Lu-PSMA-617 in the VISION sub-study patient population were consistent with previously published estimates. The mean tumor density results from cycle 1 were consistent with the ranges previously published for patients with mCRPC treated with 177Lu-PSMA-617 using SPECT imaging alone or planar imaging alone, as well as hybrid imaging procedures. Of all analyzed tumors, 96% fell within the previously reported radiation-absorbed range of tumor doses
- In addition to the good safety profile and low radiotoxicity in at-risk organs in the VISION sub-study, the present tumor dosimetry data provide further support for adoption of 177Lu-PSMA-617 as a treatment option in clinical practice for patients with mCRPC
Presented by: Prof. Bernd J. Krause, Rostock University Medical Center, Rostock, Germany
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.
References:
- Sartor O, de Bono J, Chi KN et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021 Sep 16;385(12):1091-1103.


