(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting featured a session on prostate cancer, and a presentation by Dr. Karim Fizazi discussing results from PSMAfore, specifically health-related quality of life and pain among taxane-naïve metastatic castration-resistant prostate cancer (mCRPC) patients treated with 177Lu-PSMA-617. In the VISION trial, 177Lu-PSMA-617 plus standard of care prolonged overall survival, radiographic progression-free survival, and time to health-related quality of life/worsening pain in patients with advanced mCRPC.1-2
Previously presented at ESMO 2023, 177Lu-PSMA-617 prolonged radiographic progression-free survival versus change of androgen receptor pathway inhibitor in taxane-naïve patients with mCRPC in PSMAfore. At the ASCO 2024 annual meeting, Dr. Fizazi and colleagues presented health-related quality of life and pain outcomes at the second interim analysis.
Eligible patients had mCRPC, were candidates for change of androgen receptor pathway inhibitor after progression on one prior androgen receptor pathway inhibitor, and had ≥1 PSMA-positive and no exclusionary PSMA-negative metastatic lesions by 68Ga-PSMA-11 PET/CT. Ineligible patients were candidates for PARP inhibition or had received prior systemic radiotherapy, immunotherapy, or chemotherapy. Patients were randomized 1:1 to open-label 177Lu-PSMA-617 (7.4 GBq/6 weeks; 6 cycles) or androgen receptor pathway inhibitor change (abiraterone or enzalutamide). Patients with confirmed radiographic progression on androgen receptor pathway inhibitor change could cross over to 177Lu-PSMA-617. The trial design for PSMAfore is as follows:
Baseline characteristics were well balanced between each arm of the trial, with a median age of 72 years (range: 43–94):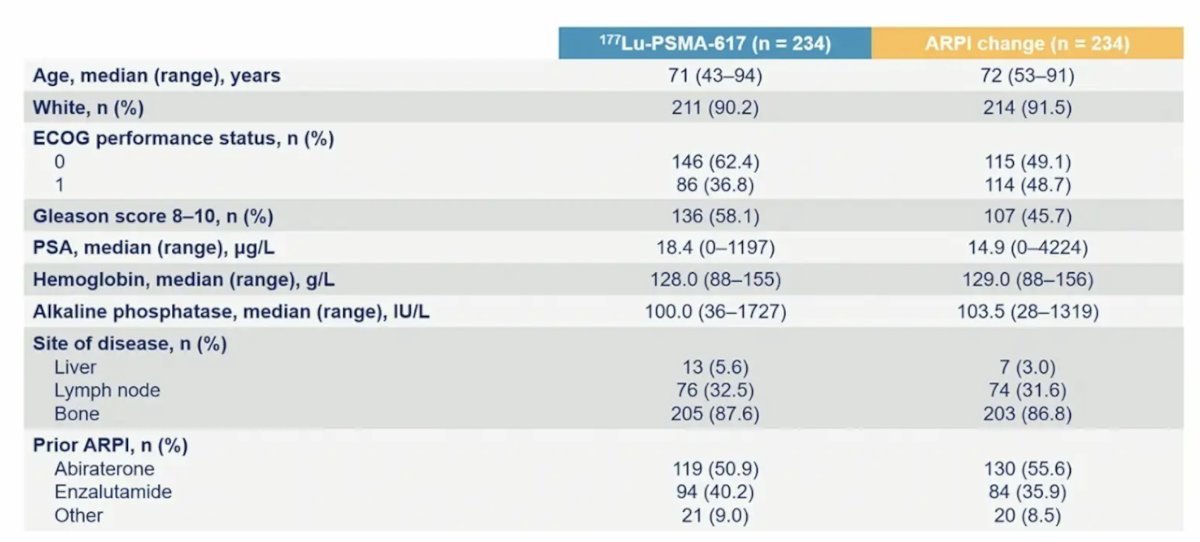
At this third interim analysis of overall survival, the hazard ratio is now < 1, with 73% information fraction in the intention to treat analysis group (HR 0.98, 95% CI 0.75-1.28):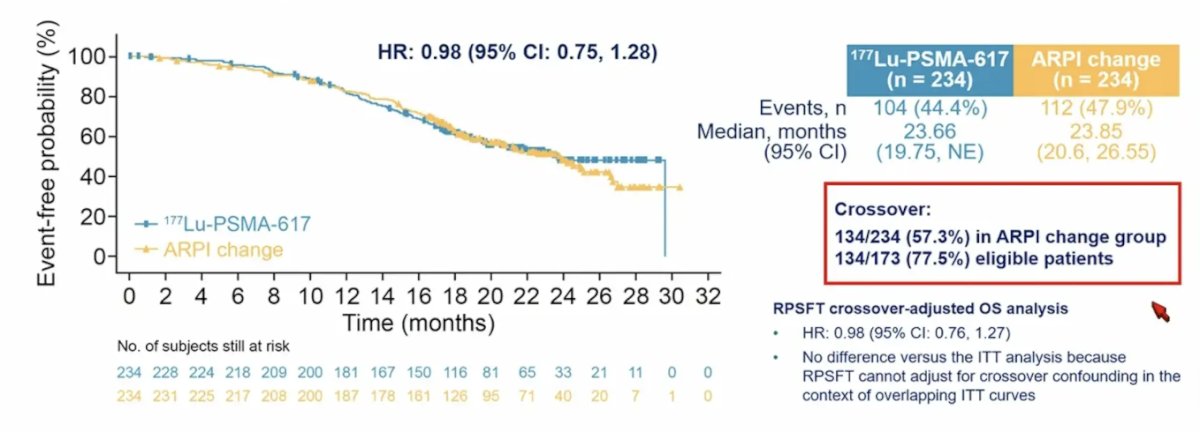
Secondary endpoints included time to worsening in self-reported health-related quality of life (FACT-P, EQ-5D-5L) and pain (BPI-SF), defined as a composite of score worsening (prespecified thresholds), clinical progression (including new anti-cancer treatment) or death. Post hoc analyses of time to worsening in FACT-P and BPI-SF excluded clinical progression and death. Of note, the study was not powered for these endpoints, and type I error was not controlled.
PSMAfore randomized 468 patients (234 per arm). Median duration of exposure was 8.4 months for 177Lu-PSMA-617 and 6.5 months for androgen receptor pathway inhibitor change. 177Lu-PSMA-617 delayed time to worsening in FACT-P (HR 0.59, 95% CI 0.47-0.72) and EQ-5D-5L (HR 0.61, 95% CI 0.50-0.76):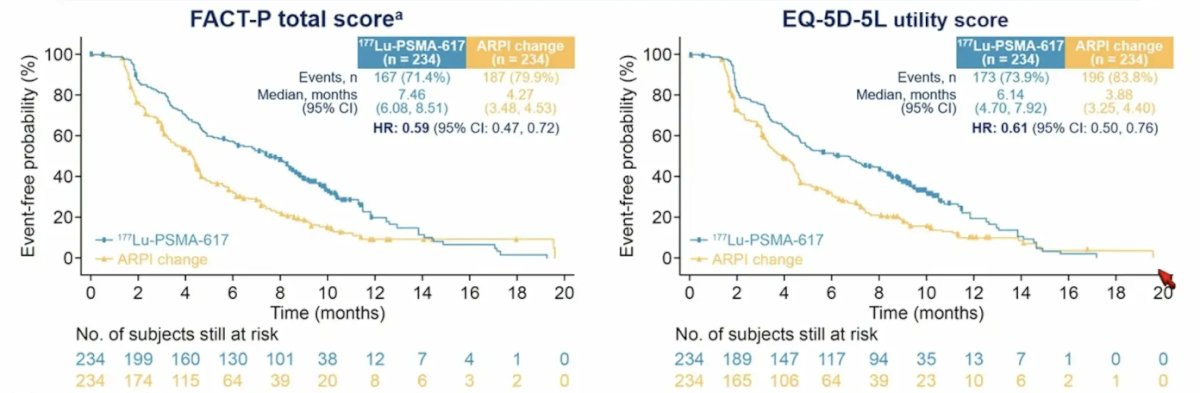
Time to worsening of the FACT-P subscales was also improved for physical well-being (HR 0.55, 95% CI 0.45-0.68), emotional well-being (HR 0.66, 95% CI 0.53-0.82), and functional well-being (HR 0.77, 95% CI 0.63-0.95), but not social/family well being (HR 0.85, 95% CI 0.69-1.06):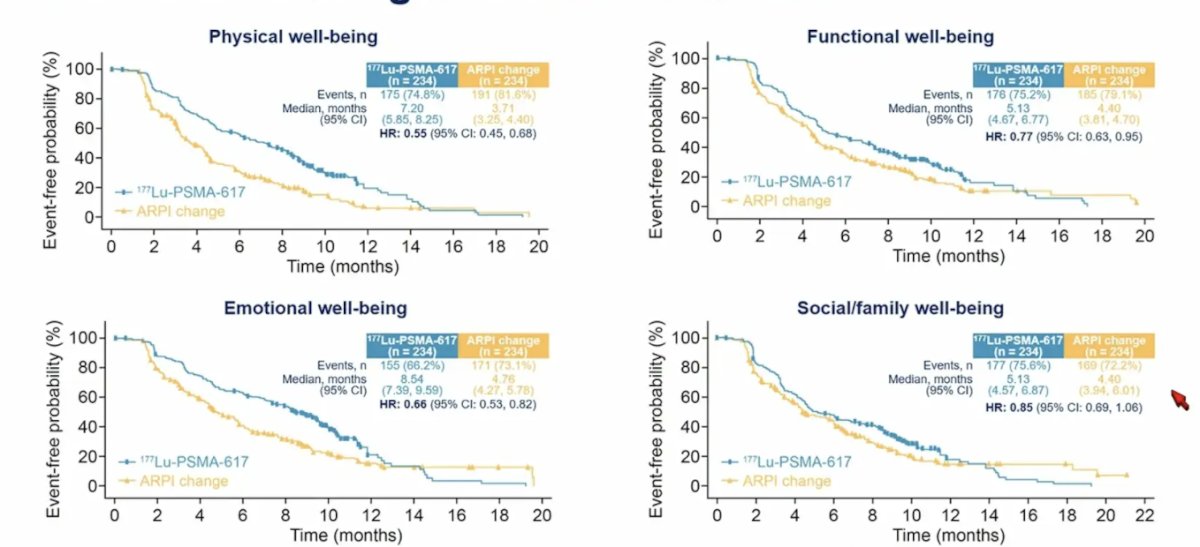
The BPI-SF scales of time to worsening pain intensity (HR 0.69, 95% CI 0.56-0.85), time to worsening pain interference (HR 0.67, 95% CI 0.54-0.83), and time to disease-related pain (HR 0.70, 95% CI 0.57-0.85) were also improved with 177Lu-PSMA-617:
When patients were excluded that had clinical progression or death, the FACT-P total score (HR 0.48, 95% CI 0.37-0.63) and BPI-SF pain intensity (HR 0.64, 95% CI 0.50-0.83) remained statistically improved for patients receiving 177Lu-PSMA-617 versus androgen receptor pathway inhibitor change: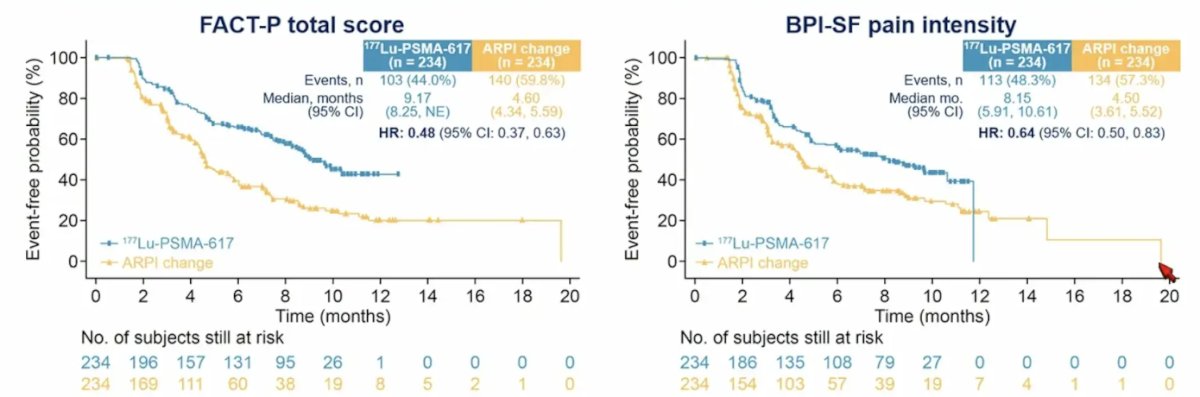
The incidence of grade ≥3 adverse events, serious adverse events, and adverse events leading to discontinuation for 177Lu-PSMA-617 and androgen receptor pathway inhibitor change were 34% vs 44%, 20% vs 28%, and 5.7% vs 5.2%, respectively. A summary of treatment-emergent adverse events is as follows: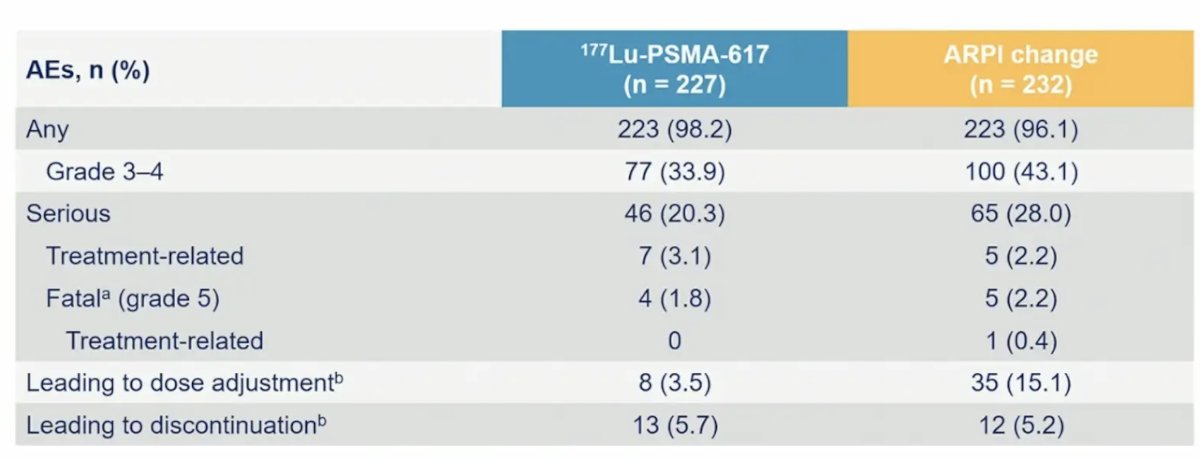
The most common treatment-emergent adverse events in >= 10% of patients in either arm included dry mouth, asthenia, nausea, anemia, and fatigue: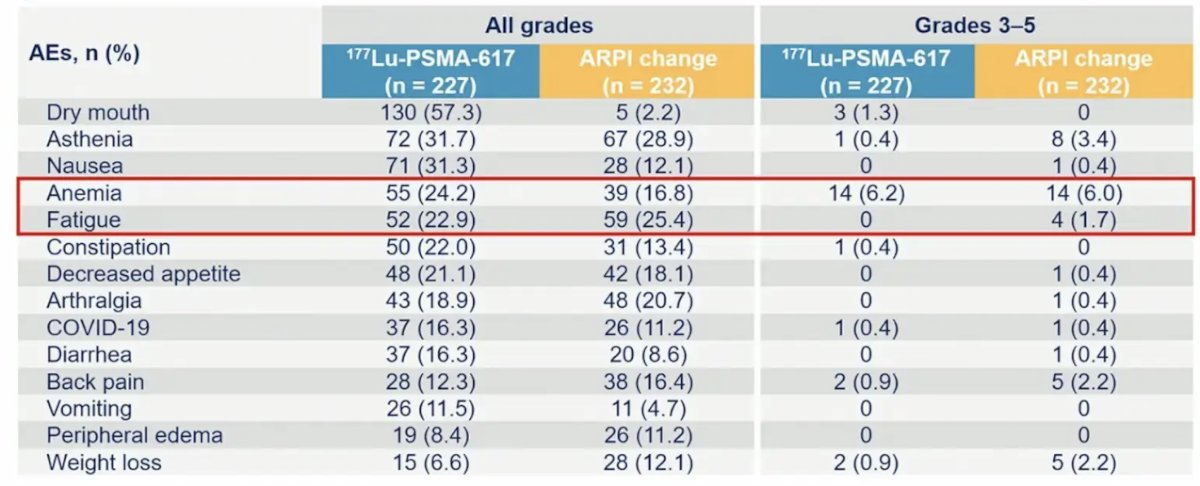
Dr. Fizazi concluded his presentation discussing health-related quality of life and pain results from the PSMAfore trial with the following take-home messages:
- 177Lu-PSMA-617 delayed cancer deterioration from the following standpoints:
- Radiographic progression-free survival: HR 0.41, 95% CI 0.29-0.56
- Health-related quality of life: FACT-P total score (HR 0.59, 95% CI 0.47-0.72), EQ-5D-5L utility score (HR 0.61, 95% CI 0.50-0.76)
- Pain: BPI-SF pain intensity (HR 0.69, 95% CI 0.56-0.85)
- 177Lu-PSMA-617 had a favorable safety profile
- Overall was similar between arms (HR 0.98 at 73% information fraction) but likely confounded by high cross-over
- Together, these data support 177Lu-PSMA-617 as a treatment option for patients with mCRPC who have undergone androgen receptor pathway inhibitor treatment
Presented by: Karim Fizazi, MD, PhD, Medical Oncologist, Institut Gustave Roussy, University of Paris-Saclay, Villeuf, France
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024.
References:
- Sartor O, de Bono J, Chi KN et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021 Sep 16;385(12):1091-1103.
- Fizazi K, Herrmann K, Krause BJ, et al. Health-related quality of life and pain outcomes with [177Lu]Lu-PSMA-617 plus standard of care versus standard of care in patients with metastatic castration-resistant prostate cancer (VISION): A multicentre, open-label, randomized, phase 3 trial. Lancet Oncol. 2023 Jun;24(6):597-610.


