(UroToday.com) The Society of Nuclear Medicine & Molecular Imaging (SNMMI) 2024 Annual Meeting held in Toronto, ON between June 8th and June 11th, 2024 was host to a prostate-specific membrane antigen (PSMA) radioligand therapy (RLT) tumor board session. Dr. Oliver Sartor discussed whether earlier use of PSMA RLT in earlier settings is of clinical benefit.
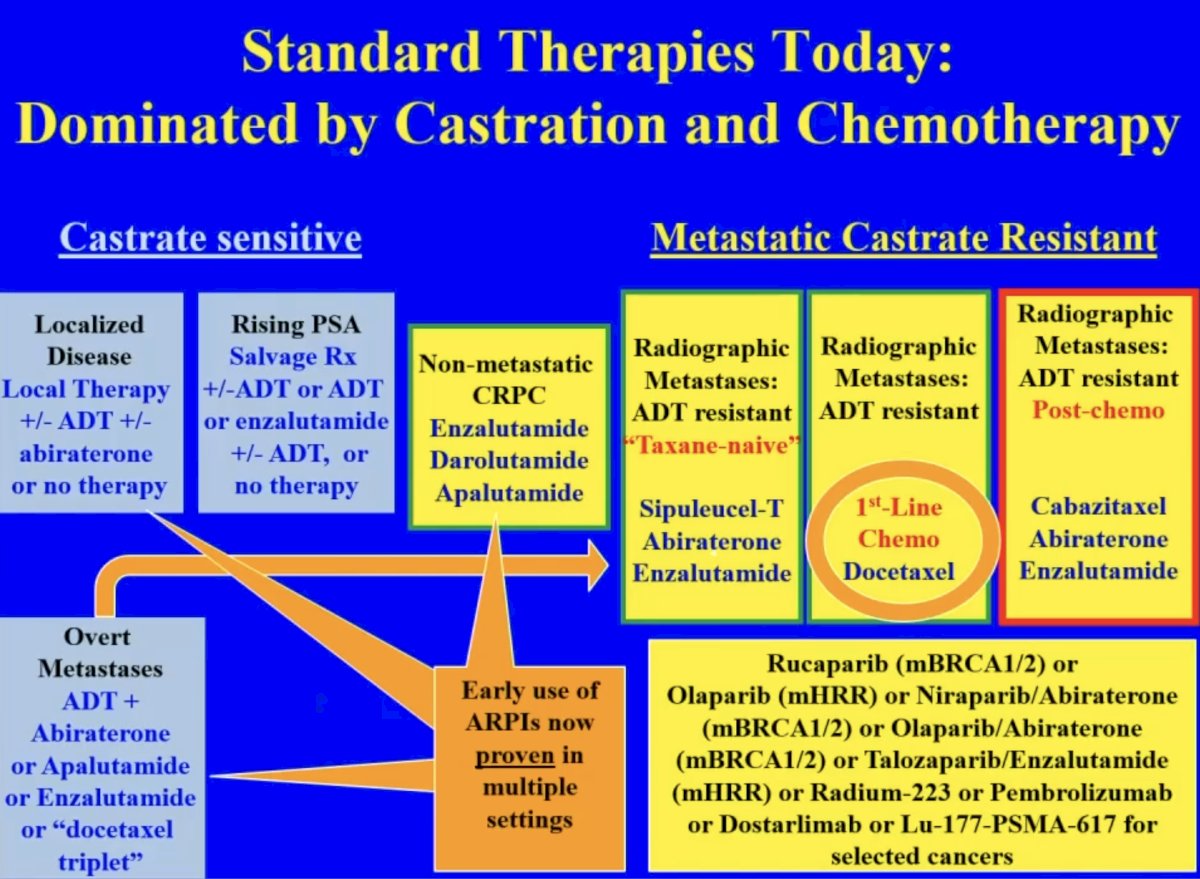
The current treatment landscape for advanced prostate cancer is dominated by castration and chemotherapy. Emerging therapies that have received regulatory approval across different settings include PARP inhibitors and LuPSMA. Dr. Sartor argued that these agents, particularly LuPSMA, are emerging as increasingly attractive options in the mCRPC post-androgen receptor pathway inhibitor treatment setting and may likely ‘move up’ into this disease space.
Some notable randomized phase 2/3 studies of LuPSMA in the mCRPC disease space include:
- TheraP and VISION (post-ARPI and post-taxane)
- PSMAfore, SPLASH, and ECLIPSE (post-ARPI, taxane-naïve)
- ENZA-p (ARPI naïve mostly)
What is the current proven benefit for LuPSMA from phase 2/3 trials? Overall survival benefits were observed in VISION.1 Radiographic progression-free survival benefits for LuPSMA were observed in TheraP,2 PSMAfore, and ENZA-p.3 Objective response rates (by cross-sectional imaging) were observed in VISION, TheraP, and PSMAfore. However, there are some ‘controversies’ and limitations to these trials and, thus, their interpretability for application in clinical practice. The control arms in VISION (standard of care therapy) and PSMAfore (ARPI switch) may be considered ‘weak’ control arms that are not currently representative of actual real-world practice patterns. In ENZA-p, patients underwent interval/serial PSMA-PET imaging for which the Prostate Cancer Working Group (PCWG3) criteria cannot be reliably applied. As such, it is important to consider these nuances when attempting to frame the benefits of LuPSMA for these patients.
When is one therapy preferred over another? Castration is associated with loss of libido, erectile dysfunction, fatigue, cognitive issues, depression, emotional lability, muscle loss, bone loss, lassitude, hot flashes, glucose intolerance, etc. Conversely, chemotherapy is associated with fatigue, loss of appetite, nausea, vomiting, diarrhea, hair loss, mouth sores, skin and nail problems, memory loss, nerve damage, increased risk of infection, etc. As such, Dr. Sartor proposed that radioligand therapy should be given prior to castration and chemotherapy, so that these agents can be delayed/avoided.
PSMAfore is a phase III trial that randomized mCRPC patients with ≥1 PMSA positive lesion and no exclusionary PSMA negative lesions by 68Ga-PSMA-11 PET/CT 1:1 to open-label 177Lu-PSMA-617 (7.4 GBq every 6 weeks for 6 cycles) or androgen receptor pathway inhibitor (ARPI) change (abiraterone or enzalutamide). Significantly, eligible patients could not be candidates for PARP inhibitor therapy and were required to be taxane-naïve or received taxane chemotherapy in the neoadjuvant or adjuvant setting >12 months ago. Patients randomized to ARPI could crossover to 177Lu-PSMA-617 following centrally reviewed radiographic progression. The study design is as follows:
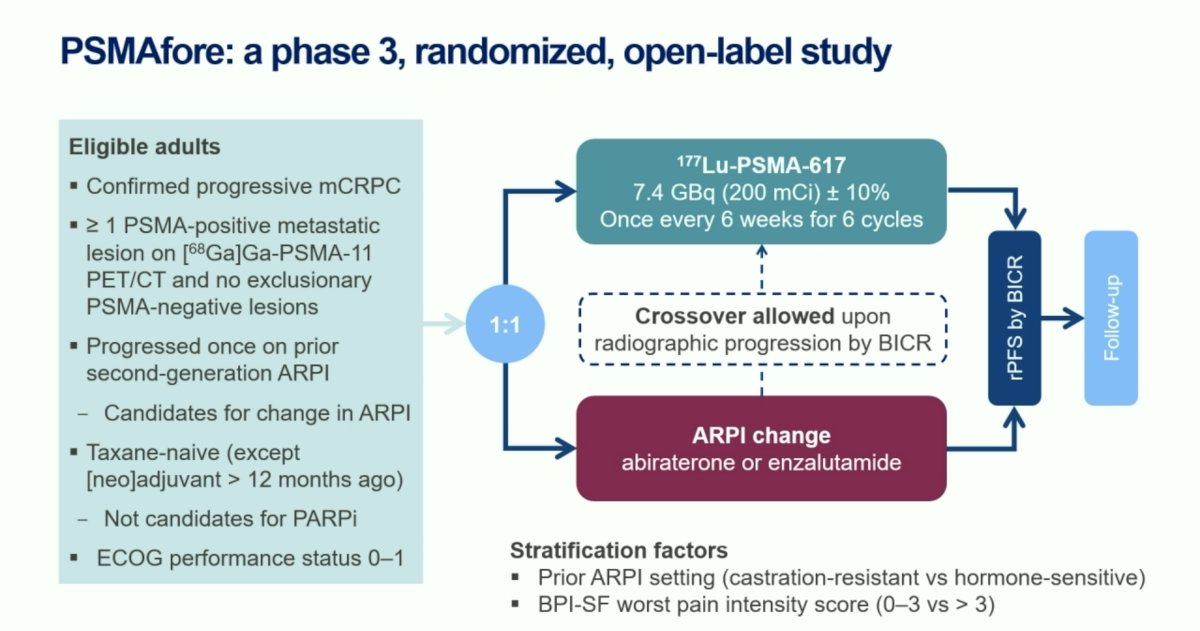
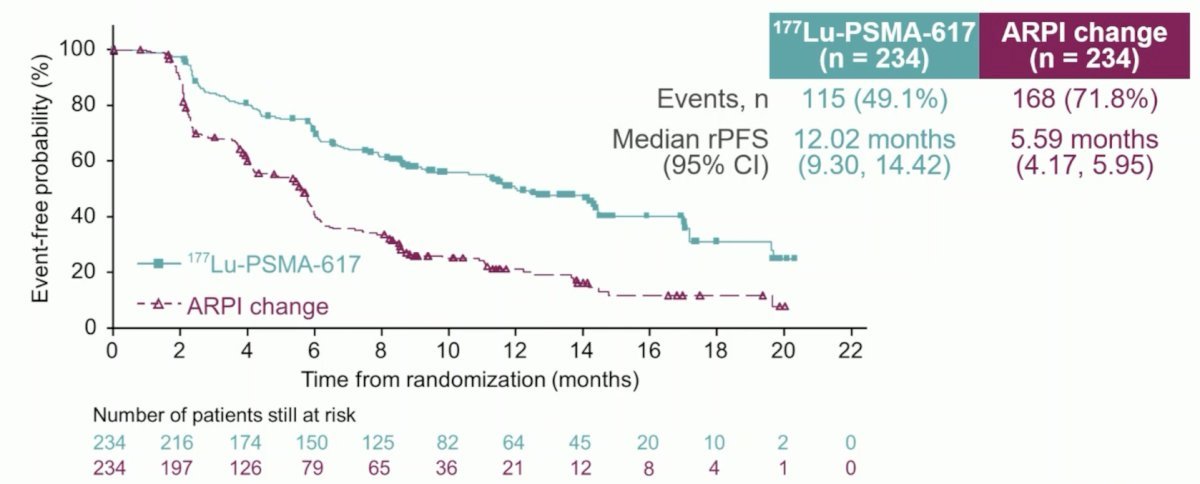
At the primary analysis (median follow-up, 7.3 months), the primary endpoint of radiographic progression-free survival was met (HR: 0.41, 95% CI: 0.29–0.56), which was similar to the results at the second interim analysis (HR: 0.43, 95% CI 0.33–0.54).
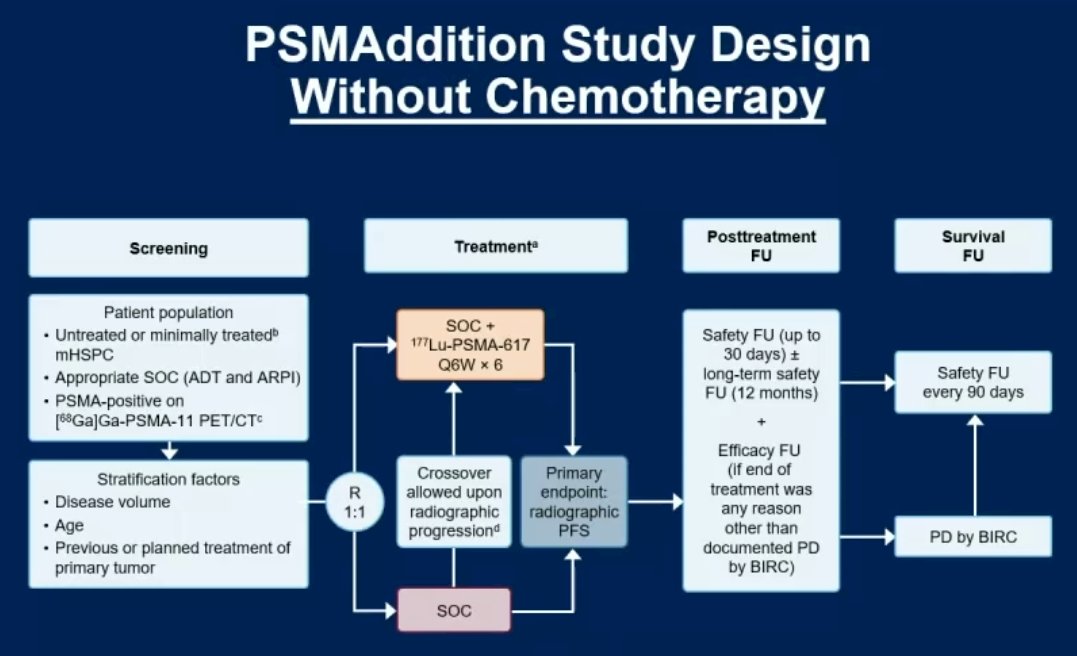
Moving to the castrate-sensitive space, PSMAddition is a phase 3 trial of 177Lu-PSMA-617 + standard of care versus standard of care alone in patients with mHSPC (n=1,226). The study design is illustrated below. Recruitment for this trial was completed in 2023, with an estimated study completion date of February 2026.
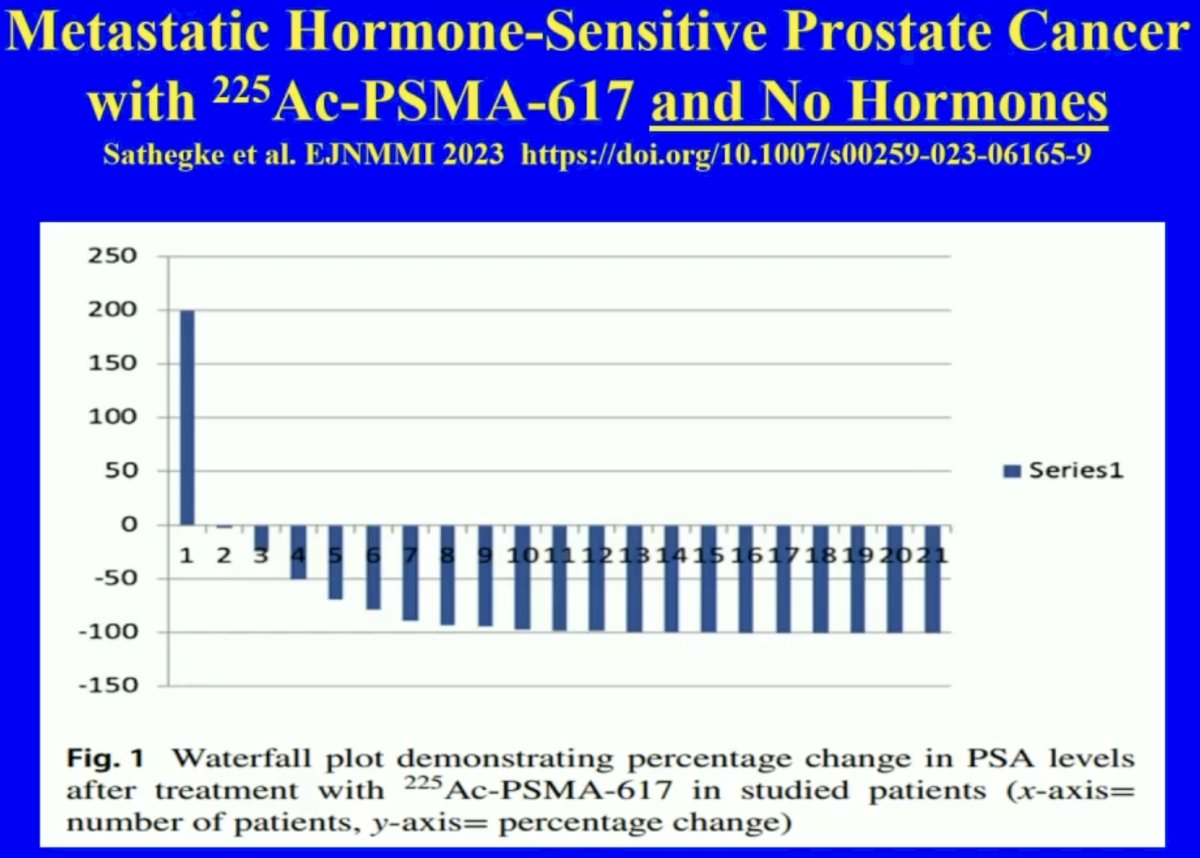
A novel approach in the metastatic castrate-sensitive setting is treatment with 225Ac-PSMA-617 monotherapy, without concurrent hormone therapy. In a retrospective analysis of 21 patients with mHSPC, 20 patients (95%) had any decline in PSA and eighteen patients (86%) presented with a PSA decline of ≥ 50% including four patients in whom PSA became undetectable.4
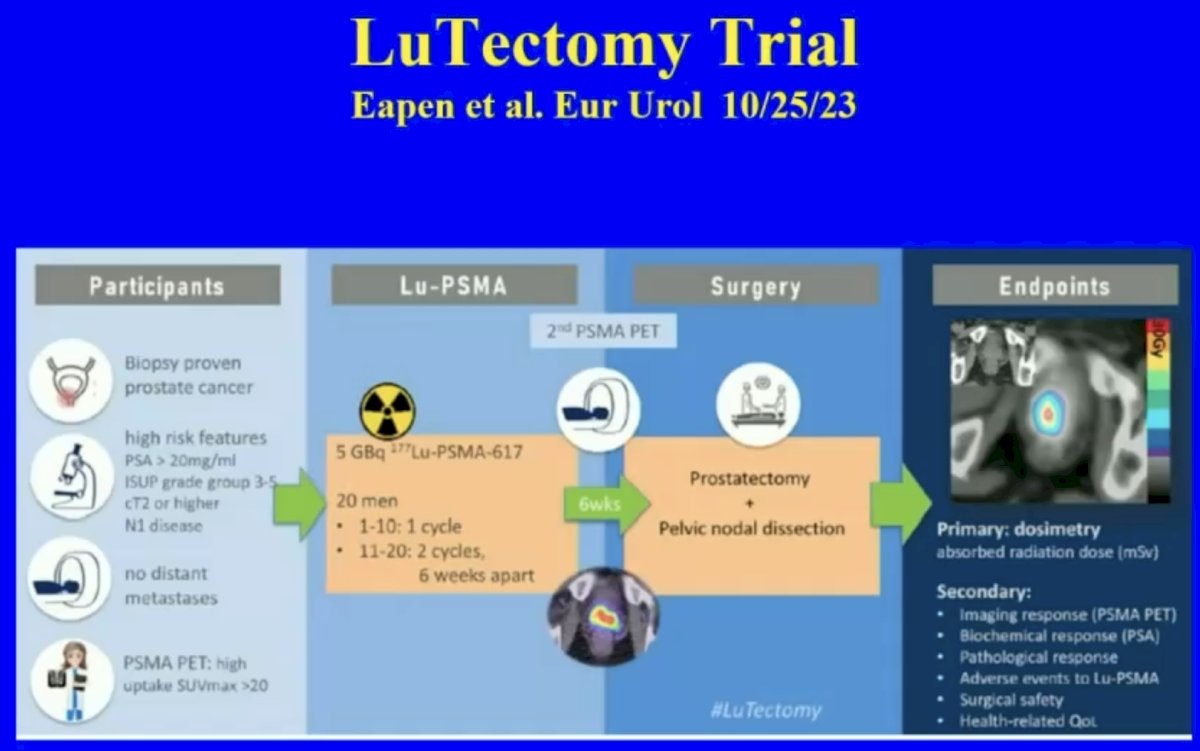
What about clinically localized disease? The phase I/II LuTectomy trial evaluated the use of 177Lu-PSMA-617 in the neoadjuvant setting prior to radical prostatectomy. Results of this trial were recently published in European Urology and demonstrated that PSA50 responses could be achieved in 9/20 patients with no observed grade 3-4 toxicities or Clavien grade 3-5 complications. From a pathologic standpoint, one patient had minimal residual disease on final histology, and no patient achieved a pathologic complete response.5
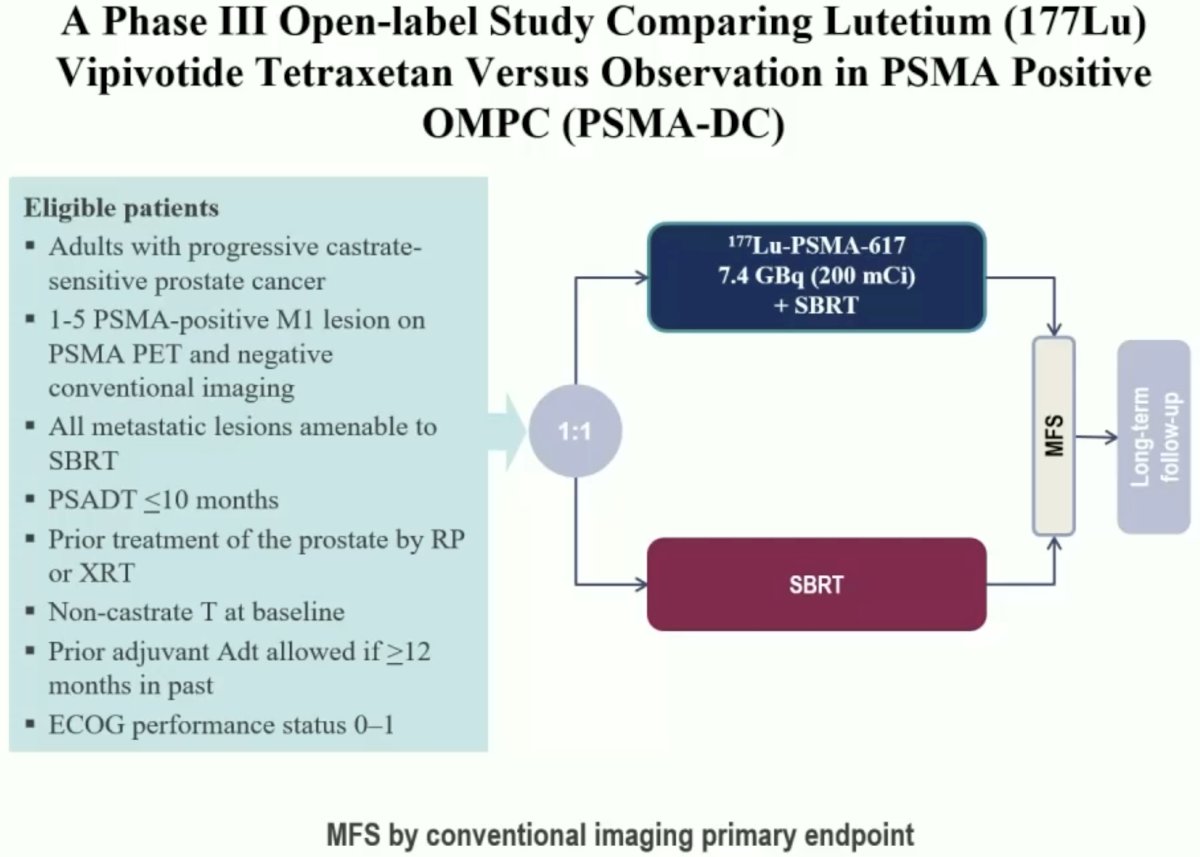
What about treating very early metastatic disease (e.g., PSMA PET positive and conventional imaging negative)? PSMA-DC (Delayed Castration) is a phase III open-label trial that is comparing 177Lu-PSMA-617 + stereotactic body radiotherapy (SBRT) to SBRT alone in metachronous mHSPC patients with a PSADT ≤10 months and 1–5 PSMA-positive M1 lesions on PSMA PET with negative conventional imaging (all lesions amenable to SBRT).
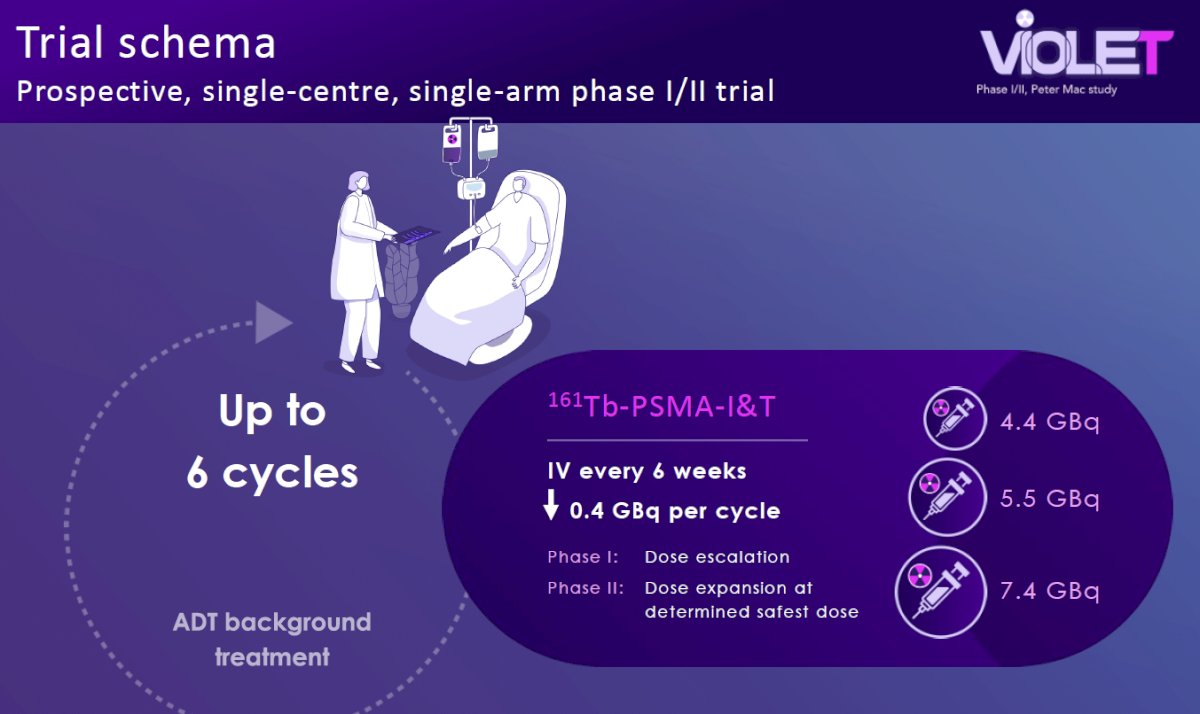
How can we overcome some mechanisms of resistance to beta-emitters, such as LuPSMA? The ideal tumor volume for beta-emitting therapies is suggested to be around 2 mm. As such, when tumors are initially treated, they may reduce to sizes that no longer confer susceptibility to this beta-emitting therapy, potentially mediating tumor resistance. VIOLET (NCT05521412) is an ongoing phase I/II trial being conducted at the Peter MacCallum Cancer Centre in Australia evaluating Terbium-161-PSMA-I&T in mCRPC patients. 161Tb has a delivers a higher beta mean energy (154.3 keV versus 133.3 keV) that may allow for the treatment of small micrometastases that may not be too small to receive a lethal dose from beta radiation alone. 161Tb attaches to PSMA receptors and emits beta particles similar to 177Lu, killing larger sized tumors with abundant crossfire. 161Tb also emits additional Auger electrons that delivers higher concentrations of radiation over very short path-lengths, which may better kill micrometastases compared to 177Lu.
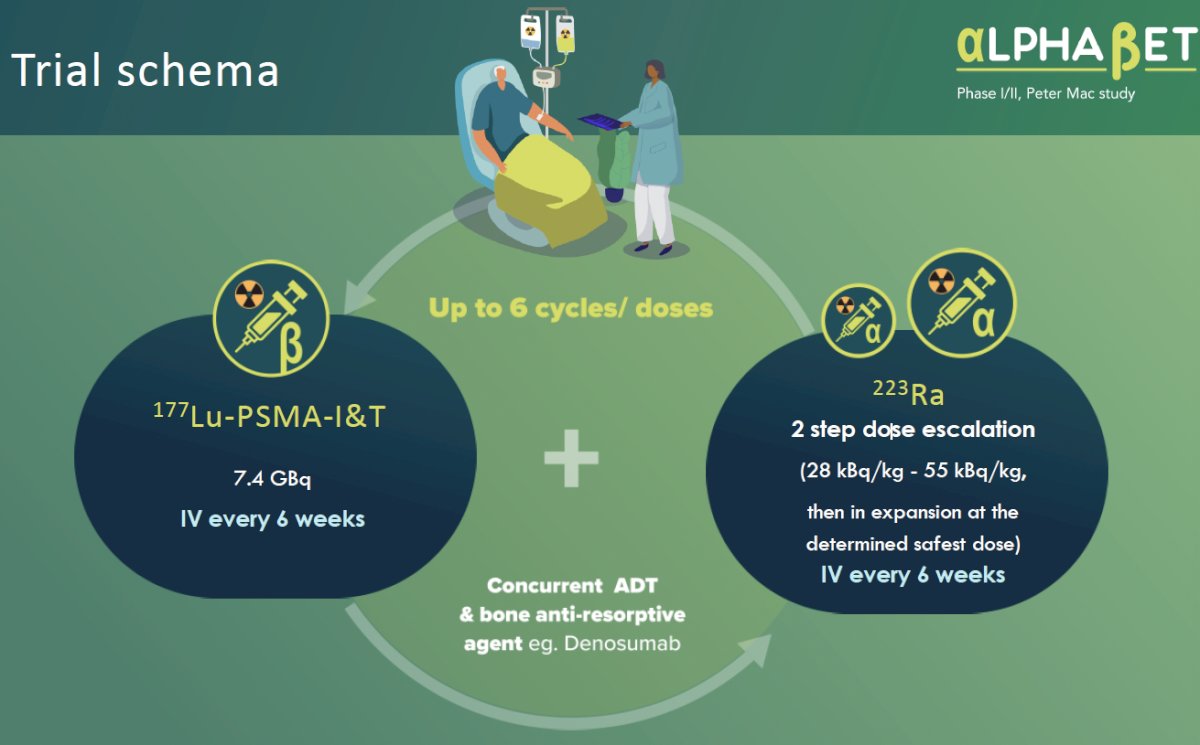
AlphaBet is a phase I/II trial evaluating the combination of Radium-223 + Lutetium-177 PSMA-I&T in mCRPC patients. 90% of patients with advanced prostate cancer develop bone metastases that can lead to pain, fractures, and spinal cord compression. 223Ra is a bone-specific calcium mimetic that emits high energy alpha radiation that may be better suited for targeting micrometastatic cancer cells in the bone. In AlphaBet, 36 mCRPC patients will receive up to 6 cycles of 177Lu-PSMA-I&T 7.4 GBq intravenously every 6 weeks + 223Ra in a two-step dose escalation (28 kBq/kg – 55 kBq/kg, then in expansion at the determined safest dose) intravenously every 6 weeks.
Dr. Sartor concluded by noting that the optimal time for PSMA radioligand therapy is when:
- You provide clinically meaningful improvements as compared to standard of care.
- Why you get a regulatory approval based on those endpoints.
- When you can provide a reasonable alternative to the castration/chemotherapy paradigm our patients abhor.
Presented by: Oliver Sartor, MD, Professor, Department of Medical Oncology, Mayo Clinic, Rochester, MN
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the Society of Nuclear Medicine & Molecular Imaging (SNMMI) 2024 Annual Meeting held in Toronto, ON between June 8th and June 11th, 2024
References:
- Hofman MS, Emmett L, Sandhu S, et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021;397(10276):797-804.
- Sartor O, de Bono J, Chi KN, et al. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021;385:1901-1103.
- Emmett L, Subramaniam S, Crumbaker M, et al. [177Lu]Lu-PSMA-617 plus enzalutamide in patients with metastatic castration-resistant prostate cancer (ENZA-p): an open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 2024;25(5): 563-71.
- Sathekge M, Bruchertseifer F, Vorster M, et al. 225Ac-PSMA-617 radioligand therapy of de novo metastatic hormone-sensitive prostate carcinoma (mHSPC): preliminary clinical findings. Eur J Nucl Med Mol Imaging. 2023;50(7): 2210-8.
- Eapen RS, Buteau JP, Jackson P, et al. Administering [177Lu]Lu-PSMA-617 Prior to Radical Prostatectomy in Men with High-risk Localised Prostate Cancer (LuTectomy): A Single-centre, Single-arm, Phase 1/2 Study. Eur Urol. 2024;85(3): 217-226.


