ASCO 2021: Systemic Therapy Considerations After Immunotherapy Combination or Monotherapy in Advanced Renal Cell Carcinoma
First-line treatment options for renal cell carcinoma typically consists of immune checkpoint blockade (ICB) combination therapy either with two ICB agent (nivolumab and ipilimumab) or ICB blockade in combination with a VEGF tyrosine kinase inhibitor (TKI). Second-line therapies can include VEGF TKI, immunotherapy, or combinations.
The data to support single-agent VEGF TKI as second-line therapy are summarized below. These are largely retrospective studies, with the exception of the last three rows, with response rates in the 30-40% range. Some of these studies suggest that cabozantinib may be the preferred TKI in the second-line setting based on the retrospective observational cohort study from Marteau et al, which showed improved response rates and longer time to treatment discontinuation relative to other TKIs, although no overall survival advantage for cabozantinib was observed in this dataset.

The phase 3 TIVO-3 trial demonstrated a statistically significant improvement in progression-free survival for tivozanib relative to sorafenib in pre-treated RCC patients as third- or fourth-line therapy. This trial led to the FDA approval of tivozanib for relapsed or refractory advanced renal cell carcinoma.

Dr. McKay then covered the evidence for immunotherapy after first-line immunotherapy in advanced RCC. She first highlighted a retrospective multicenter cohort study (https://doi.org/10.1001/jamaoncol.2020.2169) of 69 patients who were re-challenged with ICB after receiving at least two separate lines of immunotherapy. Though no complete responses were observed, the overall response rate was 23%. The best responses to re-challenge immunotherapy were seen in patients who had the best response to initial immunotherapy.

Two other studies were then highlighted looking at combination nivolumab and ipilimumab after immunotherapy, with a 15-20% response rate, including the phase 2 FRACTION study (last row). In FRACTION, no complete responses were observed, but the disease control rate was 52%.

Adaptive trials have been conducted with the addition of ipilimumab to nivolumab in patients not responding to ICB monotherapy. Though each trial was slightly different, there was a low rate of conversion from non-response to objective response and low rates of complete response.
Finally, Dr. McKay focused on VEGF TKI combination therapies post-immunotherapy. One retrospective study suggested a response rate of 51%- and 11.6-month progression-free survival in 48 patients treated with any IO + TKI after immunotherapy. The combination of pembrolizumab and Lenvatinib was studied in a recently presented phase 2 trial. The schema for this was shown below.

There were no complete responses, but the overall response rate was 55%.

Several novel combinations are being tested post-immunotherapy. Belzutifan is a selective and potent inhibitor of HIF2-alpha which has shown an objective response rate of 25% of heavily pre-treated advanced RCC patients. Based on this data, a phase 3 randomized controlled trial testing belzutifan as subsequent line therapy is ongoing.

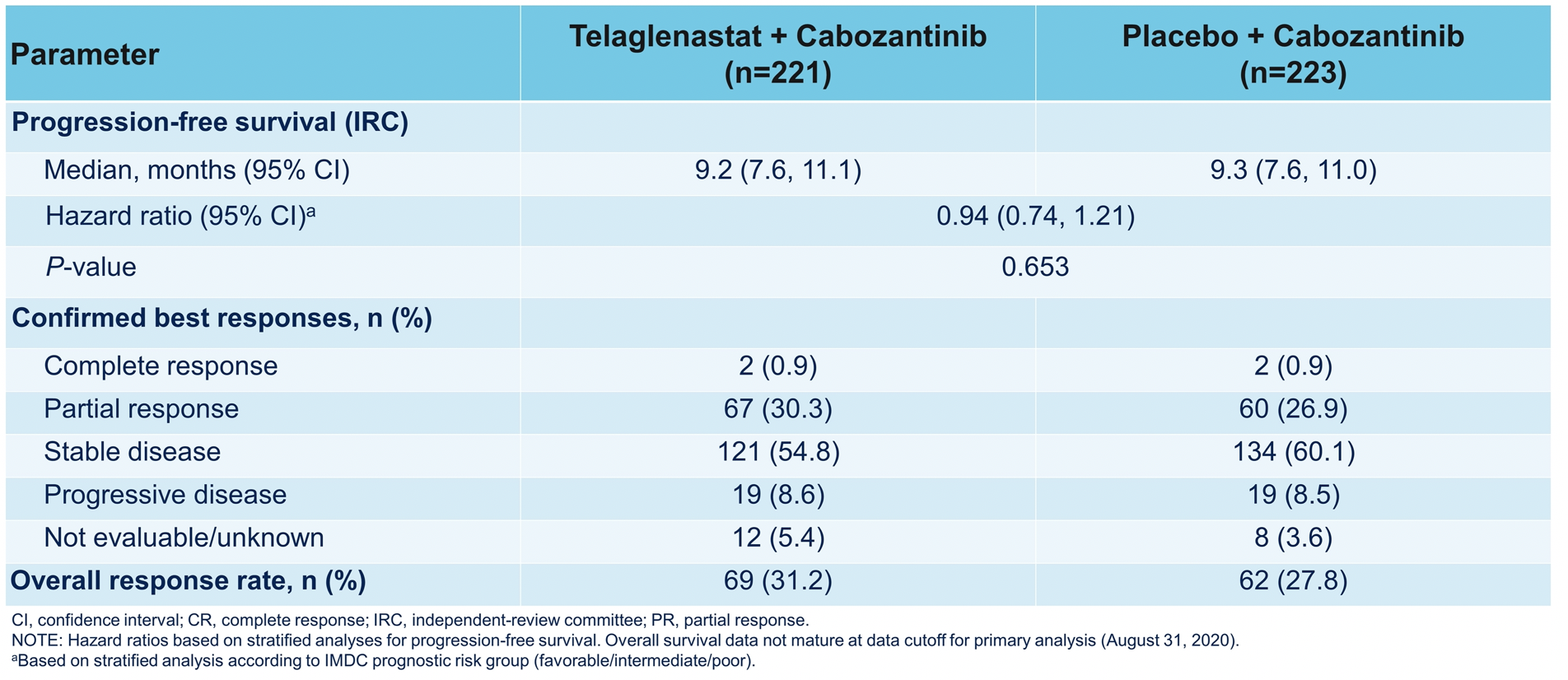
Other trials highlighted by Dr. McKay include the CONTACT-3 study of cabozantinib +/- atezolizumab in multiple histologies of advanced RCC

as well as the TiNivo-2 study of tivozanib +/- nivolumab.

Dr. McKay concluded her talk by summarizing the evidence behind multiple treatment options post-immunotherapy in advanced renal cell carcinoma. Treatment options in this context are evolving, but novel targets are needed to continue to improve outcomes for patients.

Presented by: Rana R. McKay, MD, Associate professor of medicine and urology at the University of California, San Diego in San Diego, CA
Written by: Alok Tewari, MD, Ph.D., Medical Oncologist at the Dana-Farber Cancer Institute, at the virtual 2021 American Society of Clinical Oncology Annual Meeting Congress (#ASCO21), June 4th-June 8th, 2021