(UroToday.com) The 2023 ESMO annual meeting included a trial in progress session on prostate cancer, featuring a presentation by Dr. Alicia Morgans discussing ARASTEP, a phase 3, randomized, double-blind, placebo-controlled study assessing darolutamide + ADT in patients with high-risk biochemical recurrence of prostate cancer. Up to 50% of patients with prostate cancer treated with radiotherapy and 20-30% treated with radical prostatectomy develop biochemical recurrence (BCR), defined as a PSA increase without evidence of metastases on conventional imaging. PSMA PET/CT, a more effective and precise imaging method, may detect small lesions in patients with BCR and help avoid unnecessary biopsies. Patients with BCR at high risk of metastatic progression and who have lesions identified by PSMA PET/CT need effective treatment to delay this progression. Darolutamide is a structurally distinct and highly potent androgen receptor inhibitor, which significantly improved metastasis-free survival (MFS) and overall survival (OS) in patients with nonmetastatic castration-resistant prostate cancer (CRPC),1,2 significantly improved OS in patients with metastatic hormone-sensitive prostate cancer (mHSPC),3 and showed a favorable safety profile in both settings. The ARASTEP study (NCT05794906) will evaluate whether darolutamide plus ADT improves radiological progression-free survival (rPFS) by PSMA PET/CT versus placebo plus ADT in patients with BCR following primary therapy and PSMA PET/CT-positive lesions.
Approximately 750 patients from 184 sites worldwide will be randomized to oral darolutamide 600 mg twice daily or placebo, both with ADT, for 24 months. Image-guided radiotherapy or surgery is allowed within 12 weeks from randomization. Randomization is stratified by PSA doubling time <6 vs ≥6 – <12 months, prior radical prostatectomy vs primary radiotherapy, and distant ± locoregional vs locoregional-only metastases:
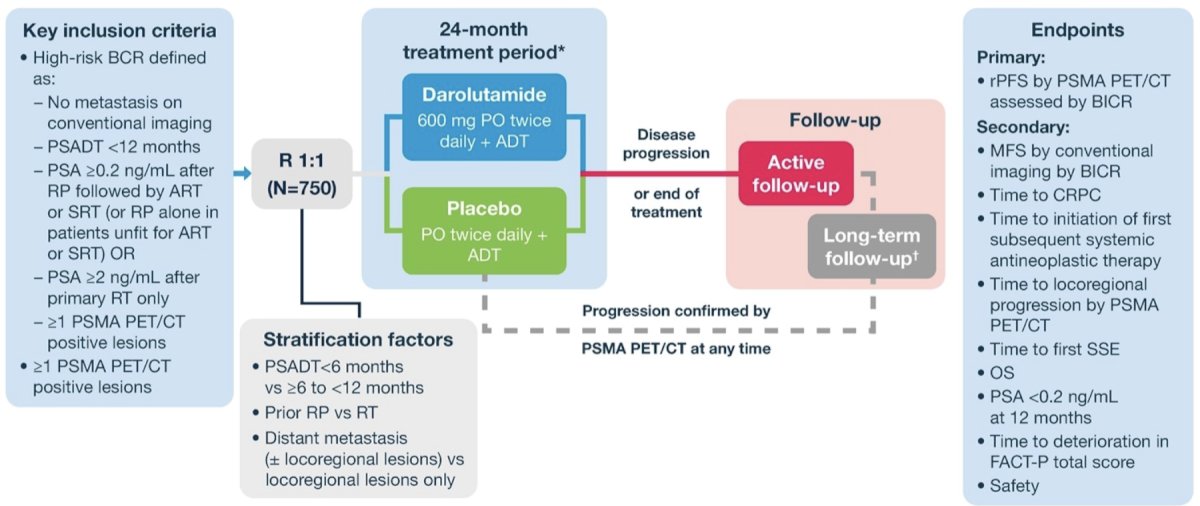
The study will comprise four consecutive periods – screening/baseline, treatment, active follow-up, and long term follow-up:

Eligible patients have prostate cancer treated by primary radiotherapy or radical prostatectomy followed by adjuvant radiotherapy or salvage radiotherapy, or radical prostatectomy alone if adjuvant radiotherapy/salvage radiotherapy was not appropriate, ≥1 PSMA PET/CT-positive lesion without visible lesions on conventional imaging, serum testosterone >150 ng/dL, with BCR. BCR is defined as:
- PSA doubling time <12 months and PSA ≥0.2 ng/mL after primary radical prostatectomy (± adjuvant radiotherapy/salvage radiotherapy) or
- PSA ≥2 ng/mL above nadir after primary radiotherapy only
The primary endpoint is rPFS by PSMA PET/CT, assessed by blinded independent central review. Secondary endpoints include MFS by blinded independent central review, time to CRPC, OS, quality of life, and safety. ARASTEP is actively recruiting and as of September 1, 2023, 1 patient has been enrolled
Presented by: Alicia K. Morgans, MD, MPH, Genitourinary Medical Oncologist, Medical Director of Survivorship Program at Dana-Farber Cancer Institute, Boston, Massachusetts
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Meeting, Madrid, Spain, Fri, Oct 20 – Tues, Oct 24, 2023.
References:
- Fizazi K, Shore N, Tammela TL, et al. Darolutamide in nonmetastatic castration-resistant prostate cancer. N Engl J Med. 2019;380(13):1235-1246.
- Fizazi K, Shore N, Tammela TL, et al. Nonmetastatic, Castration-Resistant Prostate Cancer and Survival with Darolutamide. N Engl J Med. 2020 Sep 10;383(11):1040-1049.
- Smith MR, Hussain M, Saad F, et al. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N Engl J Med. 2022 Mar 24;386(12):1132-1142.


