(UroToday.com) The 2023 ESMO annual meeting included a session on prostate cancer, featuring a presentation by Dr. Andrew Laccetti discussing the results of the phase 1/2 trial of oral EPI-7386 in combination with enzalutamide compared to enzalutamide alone in metastatic castration-resistant prostate cancer (mCRPC). The androgen receptor is activated by androgen binding to the ligand binding domain which induces the dimerization and nuclear translocation of the androgen receptor.
Current androgen receptor targeted therapies work directly or indirectly through the ligand binding domain of the androgen receptor either by competing with androgen binding to the ligand binding domain or by inhibiting the androgen production (centrally through CYP17 inhibition). EPI-7386 is a next generation antiandrogen designed to inhibit androgen receptor activity by binding the N-terminal domain and blocking transcription despite resistance driven by point mutations and splice variants in the ligand-binding domain. In preclinical models, the combination of EPI-7386 with enzalutamide results in a deeper blockade of the androgen receptor pathway and greater antitumor activity, prompting the current trial.
This phase I/II multicenter, open-label clinical trial is enrolling mCRPC patients on ADT and naïve to second-generation antiandrogens (one line of prior chemotherapy in the metastatic hormone sensitive setting allowed). Phase 1 examines escalating doses of EPI-7386 with enzalutamide, with primary and secondary endpoints of phase 1 evaluating the safety and pharmacokinetics of EPI-7386 and enzalutamide when co-administered to establish recommended phase II combination doses and address possible drug-drug interactions. Once recommended phase II combination doses are established, phase II will commence as a two arm, 2:1 randomized trial evaluating antitumor activity of EPI-7386 in combination with enzalutamide versus enzalutamide alone. The phase I and phase II trial designs are as follows:
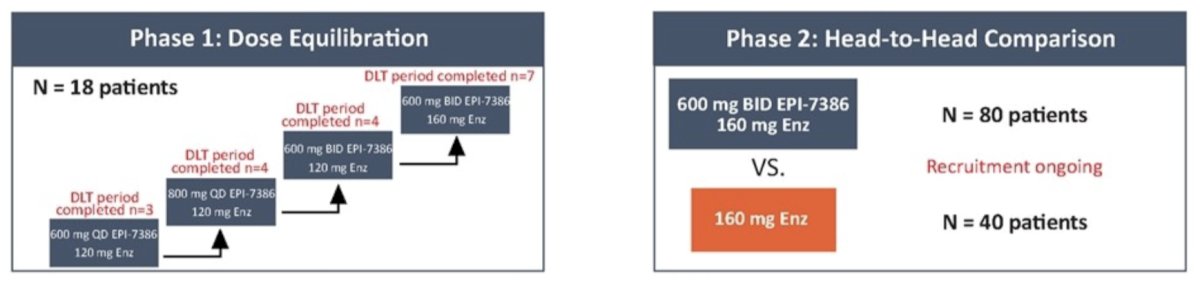
To date, 11 patients have been enrolled in cohorts 1-3, with cohort 4 currently enrolling and with 7 patients at the time of presentation (total = 18 patients). The baseline characteristics for these patients are as follows:

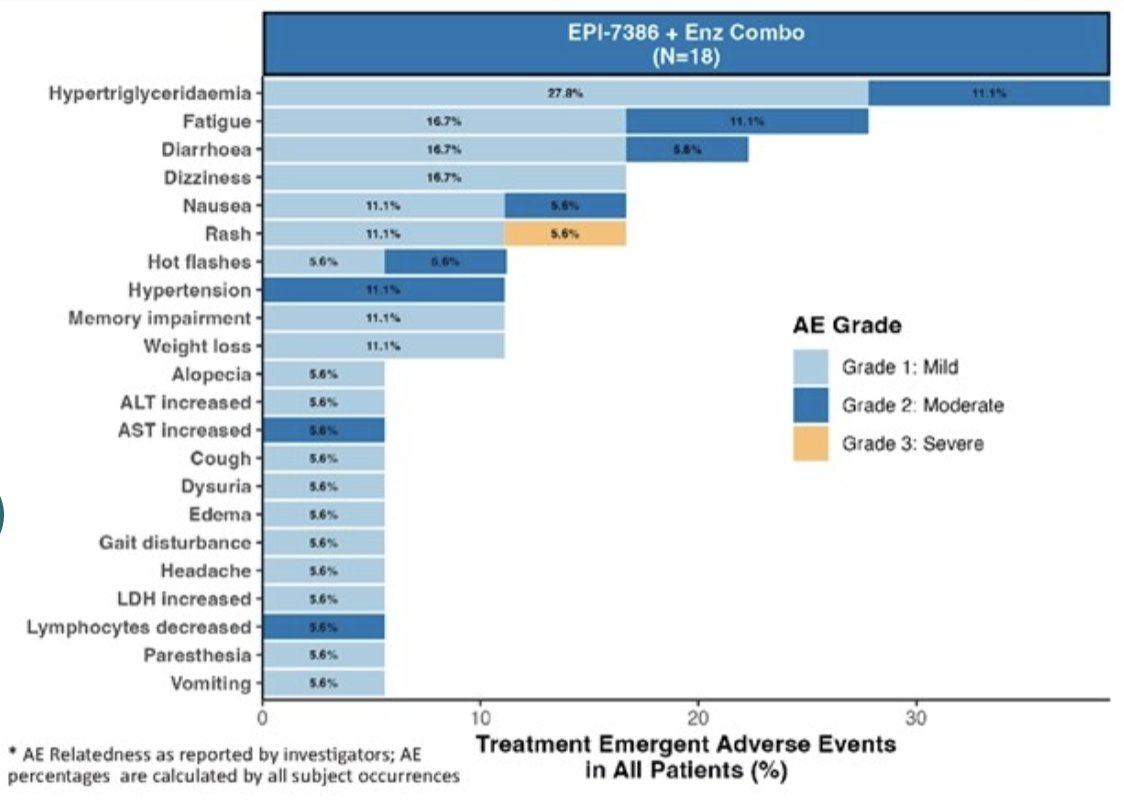
Safety is consistent with second-generation antiandrogens (ie. Grade 1 or 2 adverse events of fatigue and hot flashes). In Cohort 4, one grade 3 rash event was deemed as probably treatment related, as it was observed after administration of EPI-7386 and enzalutamide in combination during the dose limiting toxicity period:
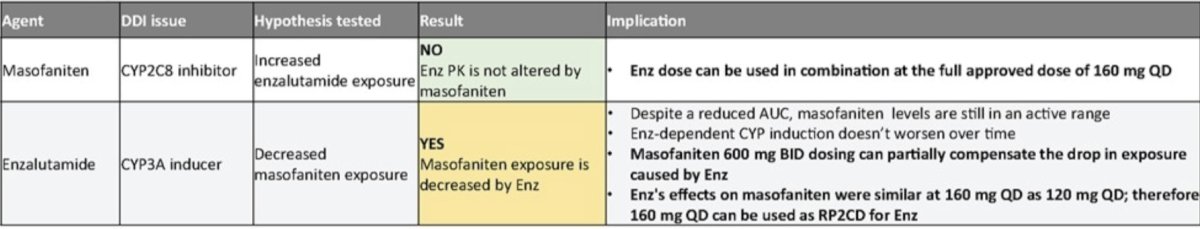
Pharmacokinetic results demonstrate enzalutamide exposure is minimally impacted by EPI-7386, allowing testing of the full dose of enzalutamide (160 mg) in the current cohort 4. EPI-7386 exposure is strongly reduced by enzalutamide (CYP3A4 inducer that metabolizes EPI-7386) but remains in the clinically relevant range seen in xenograft studies. EPI-7386 BID dosing shows an increase in EPI-7386 exposure and mitigates drug-drug interaction caused by enzalutamide:
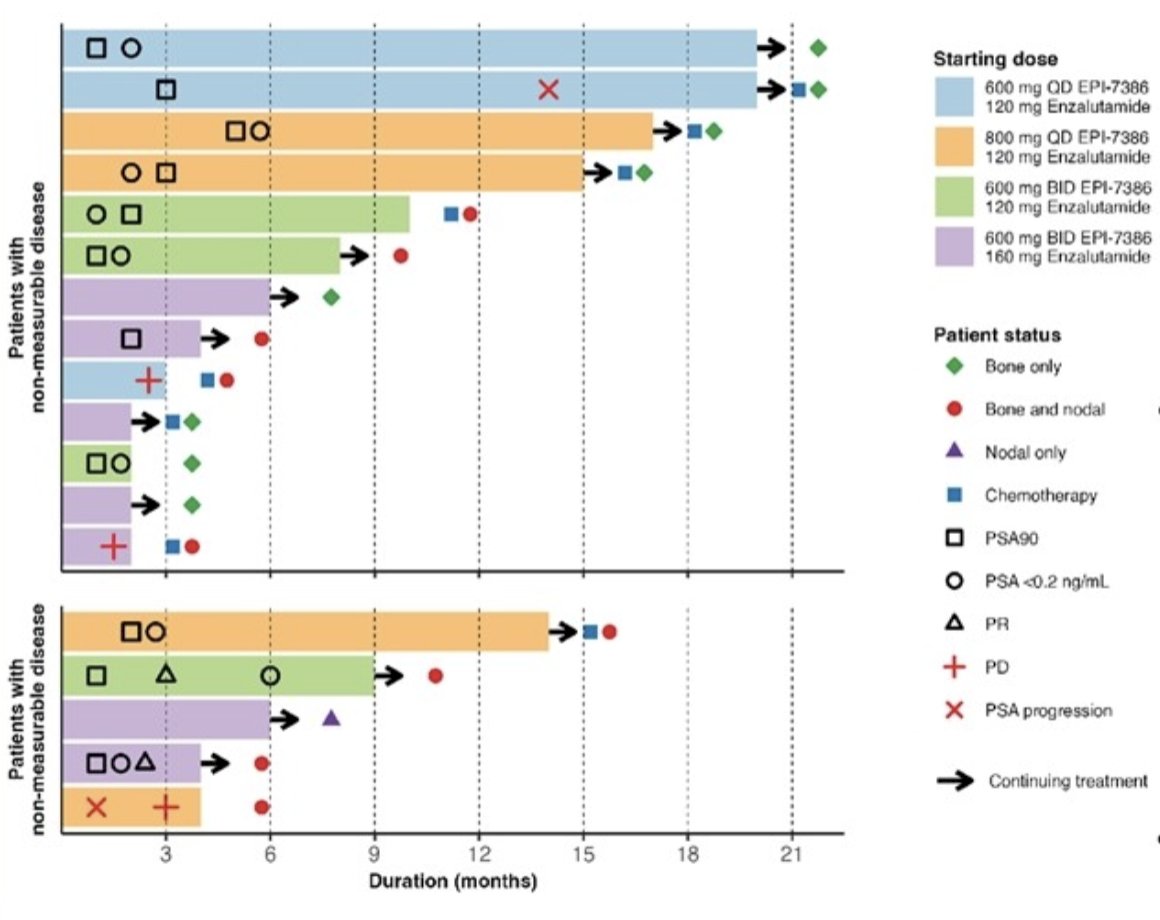
With regards to patient disposition, among 18 patients, 13 have ongoing treatment and 5 have discontinued therapy (disease progress n = 3, brain abscess n = 1, non-cancer related death n = 1). Additionally, 13 patients have non-measurable disease: bone only or bone + non-target lesions (11/13 stable disease; 2/13 progressive disease). Five patients have measurable disease:
Current response rates in evaluable patients is as follows:
- PSA50: 88%
- PSA90: 69%
- PSA < 0.2 ng/mL: 56%
Dr. Laccetti concluded his presentation by discussing the results of the phase 1/2 trial of oral EPI-7386 in combination with enzalutamide compared to enzalutamide alone in mCRPC with the following concluding statements:
- Based on the tolerability of the safety and the pharmacokinetic data from phase 1, the recommended phase 2 combination doses are 600 mg BID for EPI-7386 + 160 mg daily for enzalutamide
- Epi-7386 had no effect on enzalutamide exposure, thus allowing the use of full dose per label of enzalutamide in combination
- Enzalutamide significantly reduces EPI-7386 exposure, but BID dosing of EPI-7386 can mitigate the drop and maintain clinically relevant levels
- Rapid, deep and tolerable PSA reductions were observed in patients, regardless of previous chemotherapy status and lower than full dose enzalutamide
- To date, 69% of the patients dosed with EPI-7386 + enzalutamide achieved a PSA decline > 90%, and PSA90 was also achieved in < 90 days in 63%
- The phase 2 study is currently enrolling patients
Presented by: Andrew L. Laccetti, MD, MS, Memorial Sloan Kettering Cancer Center, New York, NY
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Meeting, Madrid, Spain, Fri, Oct 20 – Tues, Oct 24, 2023.


