(UroToday.com) The 2023 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between June 2nd and June 6th was host to a kidney and bladder cancers poster session. Dr. Rana McKay presented the rationale and study framework for SAMURAI, a randomized phase II trial evaluating stereotactic ablative radiation therapy (SABR) for patients with metastatic, unresected renal cell carcinoma (RCC) receiving systemic immunotherapy.
The role of cytoreductive nephrectomy for patients with metastatic RCC has come into question following the publication of the results of SURTIME and CARMENA.1,2 This matter is further complicated by the fact that the initial trials demonstrating efficacy for cytoreductive nephrectomy were performed in the era of interferon systemic therapy3 and the SURTIME and CARMENA trials, both negative trials for cytoreductive nephrectomy, were performed with sunitinib as the systemic treatment of choice. Given the current shift to immune checkpoint inhibitor (ICI) combination treatment strategies, the validity and transferability of the results of these studies to current practice have come into question. Nevertheless, there has been a shift towards initiation of upfront systemic therapy rather than immediate cytoreductive nephrectomy.
A proposed alternative to cytoreductive nephrectomy is SABR, which is a radiation technique that delivers a high radiation dose over a limited number of fractions. The SAMURAI trial aims to evaluate SABR as an alternative approach to treat the primary tumor in patients with metastatic RCC receiving ICI-based combinations and who are not recommended for surgery or who decline surgery.
Eligibility criteria for this study are as follows:
- Any histology RCC who are candidates to receive standard ICI combination treatment and are not recommended for upfront surgery or decline surgery
- Have a primary tumor treatable with SABR
- Choice of the ICI combination will be at the discretion of the treating physician and will include either ICI-ICI or ICI in combination with a vascular endothelial growth factor receptor (VEGFR) inhibitor
Patients in this study will be randomized 2:1 to either SABR to the kidney (42 Gy in 3 fractions) + standard immunotherapy (n=160) versus standard immunotherapy alone (n=80). Randomization will be stratified by IMDC risk (intermediate versus poor risk), planned immunotherapy type (ICI-ICI versus ICI-VEGF), and histology (clear cell versus non-clear cell).
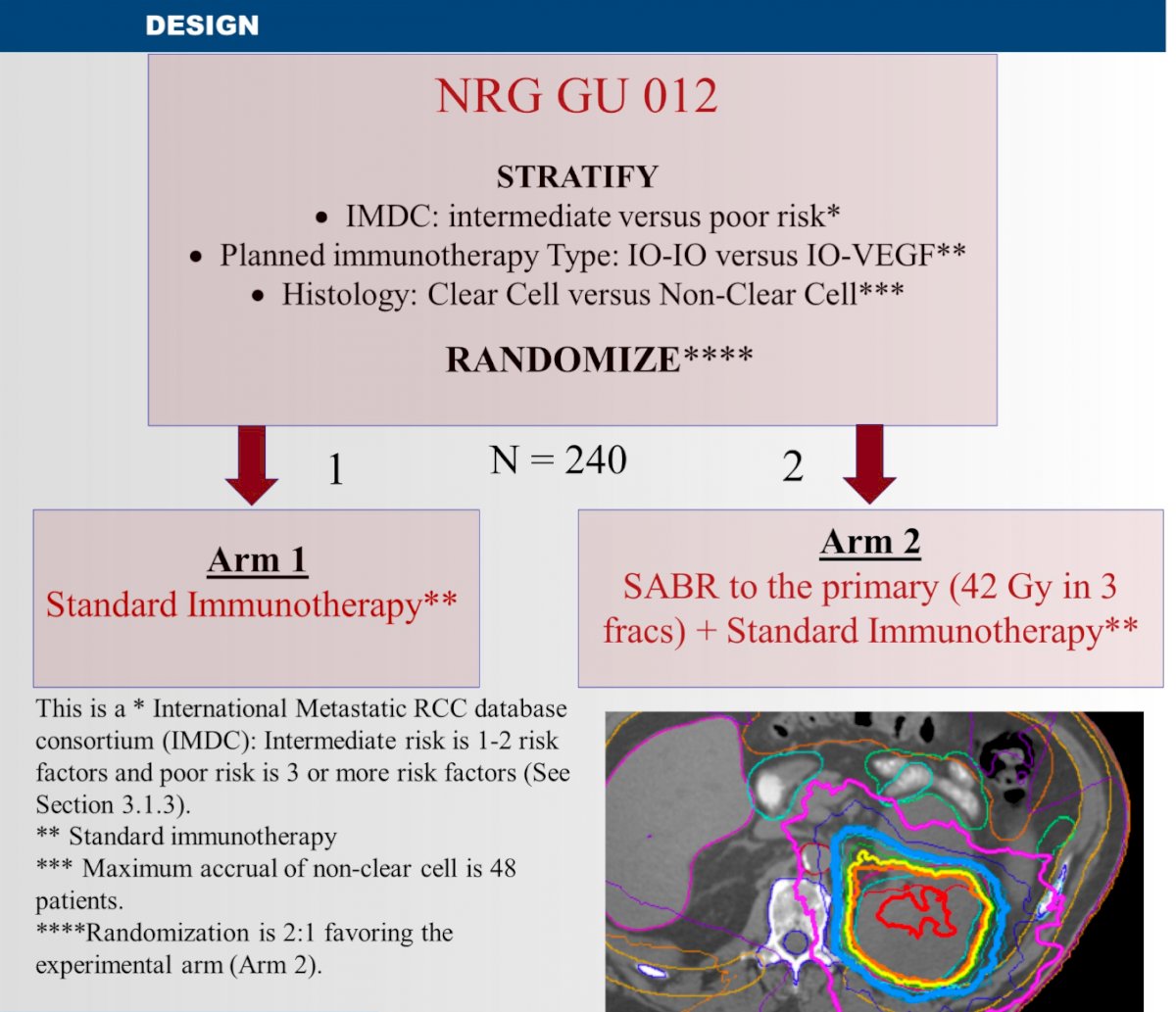
The primary study endpoint will be nephrectomy and radiographic progression-free survival (nrPFS), with progression determined as per iRECIST criteria. Secondary endpoints will include:
- Objective response rate, by iRECIST and RECIST
- Radiographic progression-free survival, by iRECIST and RECIST
- Time to 2nd line therapy
- Rate of cytoreductive nephrectomy
- Treatment-free survival
- Overall survival
- Exploratory analysis assessing potential prognostic and predictive biomarkers
The total sample size of 240 patients will provide 90% power at a 1-sided α level of 0.05 to detect an HR of 0.62 for nrPFS. Accrual of non-clear cell patients will be capped at 48. The trial is currently open and accruing through the NRG oncology cooperative group (NCT05327686).
Presented by: Rana McKay, MD, Associate Professor, Department of Medicine, University of California, San Diego, CA
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.References:
- Bex A, Mulders P, Jewett M, et al. Comparison of immediate vs deferred cytoreductive nephrectomy in patients with synchronous metastatic renal cell carcinoma receiving sunitinib: The SURTIME Randomized Clinical Trial. JAMA Oncol 2019;5(2):164-170.
- Mejean A, Ravaud A, Thezenas S, et al. Sunitinib alone or after nephrectomy in metastatic renal cell carcinoma. N Engl J Med 2018;379(5):417-427.
- Flanigan RC, Mickisch G, Sylvester R, et al. Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis. J Urol 2004;171(3):1071-1076.


