- A complete response, based on Response Evaluation Criteria In Solid Tumours (RECIST v1.1) assessment, has been reported from the first patient with metastatic castrate-resistant prostate cancer (mCRPC) to ever receive two cycles of Clarity’s 67Cu-SAR-bisPSMA at the 8GBq dose level.
- The patient remains with undetectable levels of Prostate Specific Antigen (PSA) for almost 6 months, following the administration of the second dose of 67Cu-SAR-bisPSMA. PSA is a marker used to assess clinical response to treatment and an indicator of the recurrence of prostate cancer.
- The patient had no detectable lesions using positron emission tomography (PET) imaging with 64Cu-SAR-bisPSMA following the treatment.
- The first cycle of 67Cu-SAR-bisPSMA was administered under the SECuRE trial protocol, and the second cycle under the US Food and Drug Administration’s (FDA) Expanded Access Program (EAP).
- The participant was heavily pre-treated having failed multiple lines of therapy, including androgen deprivation therapy (ADT), androgen receptor pathway inhibitors (ARPIs), chemotherapy and a poly (ADP-ribose) polymerase (PARP) inhibitor.
- No adverse events related to 64Cu-SAR-bisPSMA were observed. The safety profile of 67Cu-SAR-bisPSMA was favourable, with all adverse events related to the product showing improvement or resolution over time.
- No dose limiting toxicities (DLTs) have been reported in any of the patients treated in the SECuRE trial to date. The recruitment is ongoing for cohort 4, the first multi-dose cohort, at the highest dose level of 12GBq.
Reno, Nevada (UroToday.com) -- Clarity Pharmaceuticals (ASX: CU6) (“Clarity”, “the Company”), a clinical stage radiopharmaceutical company with a mission to develop next-generation products that improve treatment outcomes for children and adults with cancer, is pleased to announce that the first patient ever to be dosed with two cycles of 67Cu-SAR-bisPSMA at 8GBq achieved a complete response to treatment based on RECIST criteria.
The patient received the first cycle of 67Cu-SAR-bisPSMA as part of cohort 2 of Clarity’s theranostic trial, SECuRE (NCT04868604)1, evaluating 64Cu/67Cu-SAR-bisPSMA in patients with mCRPC, and a second cycle under the US FDA EAP, as requested by the patient’s clinician. Prior to 67Cu-SAR-bisPSMA, the patient had failed multiple lines of treatment, including hormone therapy, an investigational agent and chemotherapy.
Following the administration of the first cycle of 67Cu-SAR-bisPSMA, the patient showed a reduction of PSA level of >99%. The patient then received a second cycle of 67Cu-SAR-bisPSMA, which resulted in further reduction of his PSA to undetectable levels (confirmed by two consecutive tests) (Graph 1). PSA is a well characterised marker of tumour burden and clinical response to treatment as well as an indicator of the recurrence of disease for prostate cancer2-4. Moreover, PSA decline is an independent prognostic indicator of improved overall survival following radioligand therapy5,6.
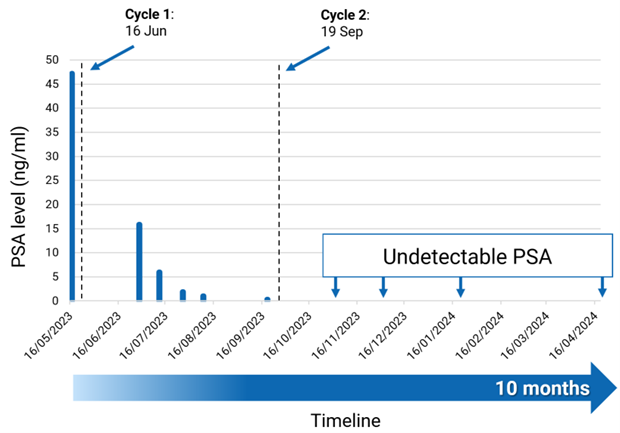
Graph 1. PSA reduction following 2 doses of 67Cu-SAR-bisPSMA (8GBq). A reduction of 99.4% in PSA was observed after the administration of the first cycle of 67Cu-SAR-bisPSMA (from the baseline of 47.2 to 0.3 ng/ml). PSA reached undetectable levels following the administration of the second cycle of 67Cu-SAR-bisPSMA. Dash lines: administration of 67Cu-SAR-bisPSMA. “Ten months” call-out in the timeline: time since the first dose of 67Cu-SAR-bisPSMA to most recent follow-up. Lower level of PSA detection: 0.05 ng/ml. Data cut off: 19 April 2024.
A complete response (absence of detectable cancer after treatment) was observed in all but one lesion assessed by computed tomography (CT) in November 2023 (one lesion showed a reduction in size from 27 mm to 12 mm, missing the complete response cut-off by only 2 mm based on RECIST assessment). No PSMA uptake was observed in any of the lesions using 64Cu-SAR-bisPSMA following the second cycle of 67Cu-SAR-bisPSMA (Figure 1).
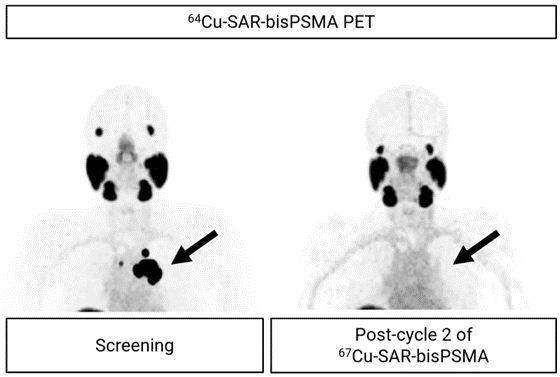
Figure 1. PET images showing uptake of 64Cu-SAR-bisPSMA in prostate cancer lesions at screening (arrow, left image; maximum standardised uptake value [SUVmax] 140.1). Image post-treatment show no 64Cu-SAR-bisPSMA uptake (arrow, right image). Images shown as maximum intensity projection.
A complete response (no detectable cancer) has now been confirmed by CT at the last follow-up (April 2024, based on RECIST assessment). The patient’s PSA remains undetectable for almost 6 months since the administration of the second cycle of 67Cu-SAR-bisPSMA (Graph 1).
No adverse events were reported as related to 64Cu-SAR-bisPSMA. Adverse events related to 67Cu-SAR-bisPSMA included dry mouth, altered taste, thrombocytopenia (all Grade 1, improved), fatigue (Grade 2, resolved) and anaemia (Grade 3, improved to Grade 2). At the last follow-up, haematological parameters were considered non-clinically significant. No DLTs have been reported in the SECuRE trial in any of the patients dosed with 67Cu-SAR-bisPSMA to date. Recruitment is ongoing into cohort 4, the first multi-dose cohort in the trial, at the dose level of 12GBq.
Dr Luke Nordquist, CEO, Urologic Medical Oncologist and Principal Investigator at the Urology Cancer Center / XCancer Omaha, NE, commented, “This was a very special moment, delivering the news to this patient that his cancer is now undetectable following the treatment with 2 doses of 8GBq of 67Cu-SAR-bisPSMA. After going through a number of therapies over the years with all of them having limited effect on the progression of his cancer, we have now been unable to detect any signs of his cancer, using PSA assessment, CT and PET imaging. The safety profile of 67Cu-SAR-bisPSMA appears to be favorable with few side effects observed following treatment, which is remarkable for a patient who was heavily pre-treated with ADT, ARPIs, chemotherapy and a PARP inhibitor.
“We are very excited to continue working with Clarity on the SECuRE trial as it has now entered a multi-dose cohort at a dose level of 12GBq, exploring the potential therapeutic benefit we might see from multiple doses of the product. The EAP has given us an early insight into what these benefits might look like, and we believe that 67Cu-SAR-bisPSMA might become a best-in-class therapeutic agent once approved, providing patients with an effective treatment option with a manageable safety profile.”Clarity’s Executive Chairperson, Dr Alan Taylor, commented, “We are very excited with this incredible response of the very first patient ever to be dosed twice at what we would consider a therapeutic dose. Our team and collaborators are encouraged by the results we are seeing with our bisPSMA product to date, and we are more dedicated than ever to continue progressing this agent through clinical trials. Seeing a patient that has gone through so many prior therapies now have undetectable disease with few side effects is extremely inspiring. Especially as we have now entered our first multi-dose cohort of the SECuRE trial, cohort 4, at a dose level of 12GBq, where we have already seen incredible benefits in patients that have failed so many lines of therapy. We hope to replicate this remarkable result in many patients and confirm the favourable safety profile of this agent.
“We believe our optimised SAR-bisPSMA product, which overcomes the issues of poor uptake and retention of current PSMA agents, combined with our dose optimisation protocol, clearly differentiates Clarity from our competitors. We hope that one day this product will become the gold standard therapeutic agent for men with mCRPC. Further enhancing our position, we recently signed a product supply agreement with NorthStar, becoming the only radiopharmaceutical company where therapeutic isotope and finished product are both centrally manufactured in the United States under one roof, solving the many manufacturing issues that have plagued our industry. This strong position has been made possible by an incredible effort from our team and collaborators and uniquely places Clarity as the major independent player in the radiopharmaceutical space.
“We will continue to focus on the rapid progression and completion of the SECuRE trial and look forward to sharing further updates as we move towards achieving our ultimate goal of better treating patients with cancer.”
References:
- Clinicaltrials.gov Identifier: NCT04868604 https://clinicaltrials.gov/ct2/show/NCT04868604
- Mandel P et al. Influence of Tumor Burden on Serum Prostate-Specific Antigen in Prostate Cancer Patients Undergoing Radical Prostatectomy. Front Oncol. 2021.
- Scher HI et al. Trial Design and Objectives for Castration-Resistant Prostate Cancer: Updated Recommendations From the Prostate Cancer Clinical Trials Working Group 3. JCO, 2016.
- Falagario UG. Biochemical Recurrence and Risk of Mortality Following Radiotherapy or Radical Prostatectomy. JAMA Netw Open. 2023.
- Rahbar K et al. PSMA targeted radioligand therapy in metastatic castration resistant prostate cancer after chemotherapy, abiraterone and/or enzalutamide. A retrospective analysis of overall survival. Eur J Nucl Med Mol Imaging, 2018.
- Ahmadzadehfar H et al. Overall survival and response pattern of castration-resistant metastatic prostate cancer to multiple cycles of radioligand therapy using [177Lu]Lu-PSMA-617. Eur J Nucl Med Mol Imaging, 2017.


