Since the 1980s, cisplatin-based chemotherapy (ChT) has been the standard of care (SoC) in the first-line (1L) setting. However, around 30% to 50% of patients are deemed ineligible for cisplatin. In cases of relapse, a non-platinum single-agent ChT (e.g. vinflunine, docetaxel, or paclitaxel) was the preferred option. In the early 2010s, five different anti-PDL1 immune checkpoint inhibitors (ICIs) underwent clinical trials in the second-line (2L) setting. In 2016, ICIs received approval from the United States Food and Drug Administration (FDA) for mUC in the platinum-refractory setting. The following year, three more ICIs were approved by the FDA. In 2017, the European Medicines Agency (EMA) approved ICIs in the 2L setting. Additionally, both the FDA and EMA granted ICIs approval as a 1L treatment for platinum-ineligible patients. In 2020, ICIs received FDA and EMA approval as maintenance therapy.
In this real-world retrospective study, Tapia et al.1 illustrate the transformative impact of ICIs on the treatment landscape of patients with mUC. There has been a change in the treatment landscape, showcasing the ascendancy of ICIs as the preferred 2L therapy, with 60% of patients receiving ICIs in this setting since 2014. This transition resulted in an approximately 30% reduction in the use of both non-platinum and platinum-based ChT. Additionally, by 2014, innovative approaches were introduced, including ICIs in the 1L treatment (18%) and targeted therapies in the 3L setting (34%). Taking together these findings, better survival outcomes for the population treated in the post-ICIs era were expected, but this did not occur. ICIs did not change the fact that only half of the patients were alive at 1 year and merely one-third at 2 years.
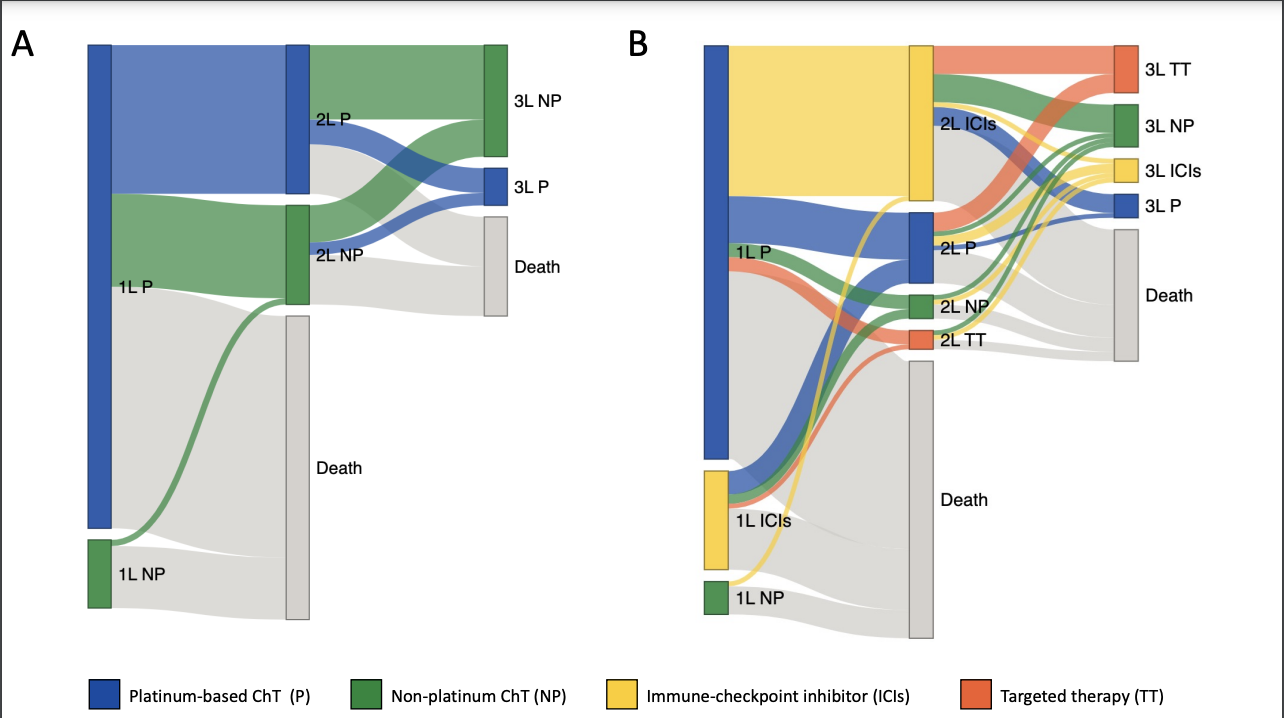
However, recent studies have demonstrated the significant impact of ICIs on overall survival (OS) when introduced earlier in the treatment landscape. First-line therapy with nivolumab plus cisplatin-based ChT has achieved an HR of 0.78 for OS (mOS: 21.7 months versus 18.9 months) when compared to cisplatin-based ChT alone.2 Nivolumab as adjuvant treatment in high-risk muscle-invasive UC has resulted in a disease-free survival (DFS) HR of 0.70 in the intention-to-treat population and an HR of 0.55 in patients with PD-L1 ≥1%.3 Finally, combination enfortumab vedotin–pembrolizumab has changed the landscape of mUC by achieving an HR of 0.47 for OS (mOS: 31.5 months versus 16.1 months) when compared to the SoC with ChT.4
In conclusion, ICIs have emerged as a transformative treatment alternative, reshaping the treatment landscape. However, substantial attrition rates from 1L to subsequent lines of systemic therapies might impede the potential impact of ICIs on long-term survival outcomes across the entire population. Although more therapeutic alternatives are available, better treatment strategies are needed in mUC.
Written by: Jose C. Tapia, MD1 and Pablo Maroto, MD, PhD2
- Velindre Cancer Center, Velindre University NHS Trust, Cardiff, Wales, United Kingdom
- Department of Medical Oncology, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain
- Tapia et al. Treatment Patterns and Survival Outcomes Before and After Access to Immune Checkpoint Inhibitors for Patients With Metastatic Urothelial Carcinoma: A Single-Center Retrospective Study From 2004 to 2021. Clin Genitourin Cancer. 2024 Feb 1;22(3):102047. doi: 10.1016/j.clgc.2024.01.019.
- van der Heijden et al. Nivolumab plus Gemcitabine-Cisplatin in Advanced Urothelial Carcinoma. N Engl J Med. 2023 Nov 9;389(19):1778-1789. doi: 10.1056/NEJMoa2309863.
- Galsky MD et al. Disease-free Survival Analysis for Patients with High-risk Muscle-invasive Urothelial Carcinoma from the Randomized CheckMate 274 Trial by PD-L1 Combined Positive Score and Tumor Cell Score. Eur Urol. 2023;83(5):432-440. doi:10.1016/j.eururo.2023.01.016
- Powles et al. Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. N Engl J Med. 2024 Mar 7;390(10):875-888. doi: 10.1056/NEJMoa2312117.


