(UroToday.com) The 2024 European Society for Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 16th, 2024 was host to a session on future therapy targets for metastatic prostate cancer patients. Dr. Juliet Carmichael discussed the potential of B7-H3 as an upcoming therapeutic target for prostate cancer.
B7-H3 (CD276) is a member of the B7 family of immunomodulatory glycoproteins, which includes PD-L1/B7-H1. Notably, PD-L1 is not expressed in most prostate cancers. B7-H3 is expressed in human placenta, but few other normal tissues. It is commonly overexpressed in prostate and other cancers, including ovarian cancer and its overexpression is correlated with worse survival outcomes. B7-HR’s functions include immune stimulation/suppression (context-dependent), tumor growth, survival, and metastasis.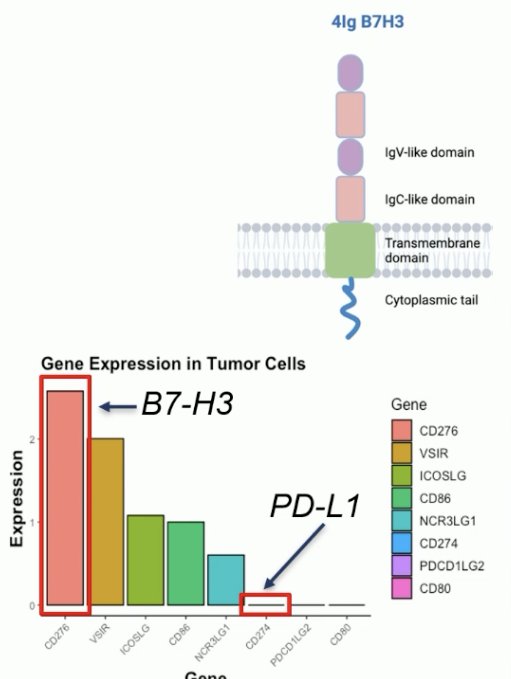
Patients with mismatch repair deficient (MMRd) tumors historically have poor prognoses, although this is now changing with the advent of immune checkpoint inhibitors which may lead to durable responses in this population. These patients are known to have higher PD-L1 expression, with 40% of MMRd mutated tumors having ≥1% PD-L1 positive cells (versus only 10% of MMR normal tumors). These patients have increased T-cell lymphocyte infiltration, which has made them a target for immune checkpoint inhibitors.1,2 
Dr. Carmichael’s lab demonstrated that B7-H3 was negatively correlated with MMRd status, which highlighted this as a potential novel, distinct tumor target to guide future treatment targeting.
Dr. Carmichael’s lab discovered that B7-H3 modulation (overexpression or knockdown) in syngenic B6CaP mouse prostate cancer organoid allograft models does not impact tumor growth.
They generated doxycycline-inducible B7-H3 overexpression in vitro (RapidCaP cell line). The growth of these B7-H3 OE cells was shown to be androgen-dependent.
These B7-H3 overexpressing cells were isolated using FACS and injected into immunocompetent C57Bl/6 mice to evaluate the impact on tumor growth, immune response, and survival.
Overexpression of B7-H3 did not influence engraftment and tumor growth of RapidCap cells in immunocompetent mice nor impact survival. Overexpression of B7-H3 did not affect RM-1 tumor growth in untreated or castrated immunocompetent mice. Taken together, these results suggest that B7-H3 may not be a key driver of tumor growth.
In 2022, Dr. Carmichael’s lab validated an immunohistochemistry assay for B7-H3 expression in CRPC tissue samples.3
Next, they evaluated B7-H3 expression in 98 CRPC biopsies, together with performing multicolor immunofluorescence to evaluate tumor immune infiltration and performing tumor genetics on a targeted panel. For 72/98 patients, there were concurrent castrate-sensitive biopsy tissue samples.
They found that B7-H3 is highly expressed from the time of primary prostate cancer diagnosis in most patients with CRPC. There was no difference in B7-H3 expression when comparing castrate-sensitive to castrate-resistant tissue.
There was no difference in B7-H3 expression between the primary and metastatic sites. Single-cell RNAseq data demonstrated that B7-H3 expression was markedly increased in tumor epithelial cells, with some concurrent expression in endothelial and immune cells.
B7-H3 was found to be expressed on tumor cells, but not the stroma. There were interspersed B7-H3 expressing and non-expressing cells, often within close proximity (20-39 µm), which has important treatment implications, given that these negative cells may be subject to a ‘bystander’ tumor-killing effect targeting the B7-H3 positive cells.
They next demonstrated that B7-H3 expression was higher in DNA damage repair (not MMRd) prostate cancer cells, including those tumors with BRCA2 biallelic mutations/homozygous deletions and those with ATM loss.
They discovered that high B7-H3 expression was associated with lower tumor-infiltrating CD3+ T cells, but higher CD4+FOXP3- T cell immune infiltration.
Next, from a clinical standpoint, there are numerous ways of targeting B7-H3 in clinical practice. These include:
- ‘Naked’ antibodies that block the receptor
- Antibody-drug conjugates
- Targeted radioligand therapy
- Bi-specific antibodies that simultaneously target B7-H3 and T cells
- Bi-specific killer engagers (BIKEs) and tri-specific killer engagers (TriKEs)
- CAR-T and CAR-NK cells

Dr. Carmichael’s lab was able to demonstrate that B7-H3 targeting with an antibody-drug conjugate with a topoisomerase-1 payload was associated with an expression-dependent anti-tumor activity in human prostate cancer cell lines.

This was further demonstrated in an in vivo B7-H3 positive prostate cancer PDX model from a patient with Gleason 9 disease and de novo high-volume disease at presentation with visceral metastases. This B7-H3 antibody-drug conjugate had anti-tumor activity in these B7-H3 positive cells. Conversely, this antibody-drug conjugate did not have activity in B7-H3 negative patients with a similar presentation.

There are currently numerous ongoing trials of B7-H3-targeted therapy for prostate cancer patients:4
Dr. Carmichael highlighted the trial of vobramitamab duocarmazine (NCT05551117) in mCRPC patients. Vobramitamab duocarmazine is a humanized anti-B7-H3 IgG1 monoclonal antibody with a topoisomerase 1 inhibitor payload. Part 1 (dose escalation) demonstrated good tolerability with early signs of anti-tumor activity. 12.0 mg/kg was selected as the dose for Part 2 (dose expansion).
Extended follow-up from Part 1 of the included mCRPC patients, with a median follow-up of 9.3 months, demonstrated that 18/54 (33%) patients achieved a partial response, despite 46% of patients having liver metastases at baseline (40% of such patients had a response). The median duration of response was 4.4 months, and seven responders remain on treatment.
TAMARACK is a randomized, open-label, global, phase II dose-selection study assessing the efficacy, safety, and tolerability of vobra duo at two dose levels (2.0 mg/kg and 2.7 mg/kg IV every 4 weeks) in mCRPC study participants. This trial demonstrated an ORR of 20% in the 2.0 mg/kg arm versus 41%in the 2.7 mg/kg arm. A PSA50 response was observed in 45% and 39% of patients, respectively. 6-month radiographic progression-free survivals were 69% and 70%, respectively.
Dr. Carmichael concluded as follows:
- Membranous B7-H3 is frequently overexpressed in CRPC. It is frequently already present at initial diagnosis and associated with DNA repair defects
- B7-H3 expression is relatively homogenous in CRPC, with positive and negative cells in close proximity
- The exact function of B7-H3 in prostate cancer and whether it is a key driver of tumour growth remains unknown
- B7-H3 is minimally expressed in normal tissue and is, therefore, an attractive therapeutic target
- B7-H3 antibody-drug conjugates have potent and selective anti-tumor activity in B7-H3 positive human CRPC models, with early signs of anti-tumor activity in trials
- B7-H3 targeting has huge promise against mCRPC
Presented by: Juliet Carmichael, MD, Institute of Cancer Research, London, United Kingdom
Written by: Rashid Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
- Rodrigues DN, Rescigno P, Liu D, et al. Immunogenomic analyses associate immunological alterations with mismatch repair defects in prostate cancer. J Clin Invest. 2018; 128(10):4441-53.
- De la Maza MDF, Chandran K, Rekowski J, et al. Immune Biomarkers in Metastatic Castration-resistant Prostate Cancer. Eur Urol Oncol. 2022; 5(6):659-67.
- Guo C, Figueiredo I, Gurel B, et al. B7-H3 as a Therapeutic Target in Advanced Prostate Cancer. Eur Urol. 2023; 83(3):224-38.
- Pulido R, Lopez JI, Nunes-Xavier CE. B7-H3: a robust target for immunotherapy in prostate cancer. Trends Cancer. 2024; 10(7):584-7.


