(UroToday.com) The 2024 European Society for Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 16th, 2024 was host to a session on future therapy targets for metastatic prostate cancer patients. Dr. Neeraj Agarwal discussed the potential applications of T-cell engagers in prostate cancer.
To date, trials of immunotherapy have failed to demonstrate meaningful survival benefits in prostate cancer patients, which are rendered immunologically inert by multiple factors, including:1
- Loss of PTEN
- Altered IFN-1 signaling
- Reduced MHC-1 expression
- Low tumor-associated antigen expression
- Low tumor mutational burden
- Decreased incidence of DNA damage repair defects
Summarized below are the major trials of immune checkpoint inhibitors for prostate cancer, which have all failed to meet their primary endpoint, except for CONTACT-02.
T-cell engagers have been proposed as a potential solution for the ‘cold’ prostate cancer immune microenvironment. How do T-cell engagers work? MHC-TCR engagement and co-stimulation is required for T-cell stimulation in normal circumstances. MHC expression is downregulated in cancer cells. T cell engagers bypass the steps needed for MHC-TCR-dependent T cell activation. T cell engagers engage both CD3 on T cells and a tumor-associated antigen on cancer cells, leading to T cell-mediated killing of cancer cells.
One of the 1st T cell engagers to be approved in oncology was blinatumomab for advanced acute lymphoblastic leukemia (ALL) based on the results of the phase III TOWER trial.
The DLL3 targeted bi-specific T cell engager, tarlatamab, was approved in May 2024 for previously treated small cell lung cancer patients based on results of the phase II DeLLphi-301 trial.
In prostate cancer, there are numerous tumor antigens that can be potentially targeted by T-cell engagers, summarized in the table below.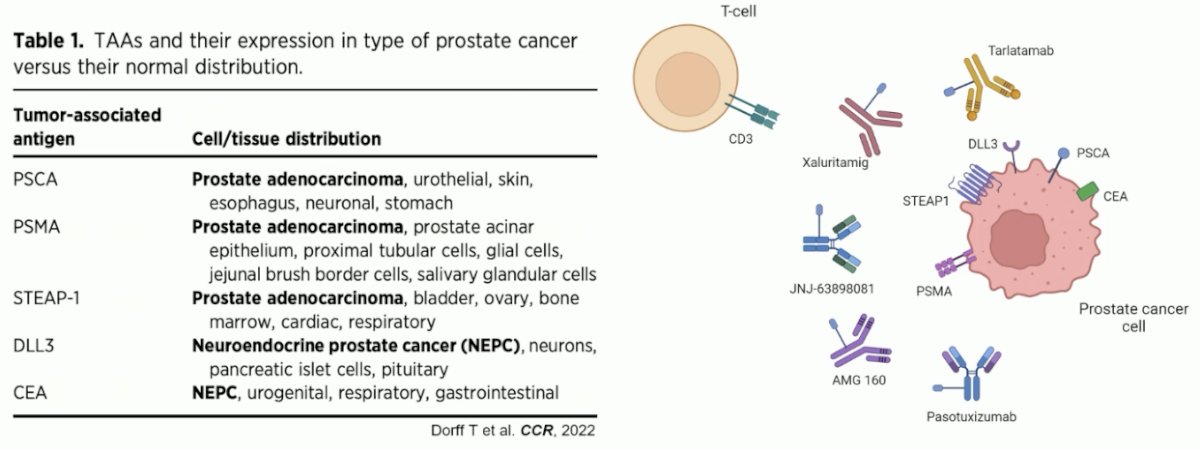
PSMA-targeting bi-specific T-cell engagers include pasotuxizumab, JNJ-63898081, acapatamab (AMG 160), and LAVA-1207.
These agents have been tested in mostly heavily pre-treated mCRPC patients with a large metastatic burden, including liver metastases. They have demonstrated variable response rates, with PSA50 responses ranging from 7.7% with JNJ-63898081 to 32% with acapatamab. One of the most common adverse events in all these trials was cytokine release syndrome, ranging in incidence from 6% to almost 100%. The onset of early antibodies led to the early discontinuation of the trials of pasotuxizumab and JNJ-63898081. Acapatamab (AMG 160) is not undergoing further development given the toxicity (cytokine release syndrome) and the lack of durable antitumor responses. LAVA-1207 is still undergoing an ongoing dose escalation study.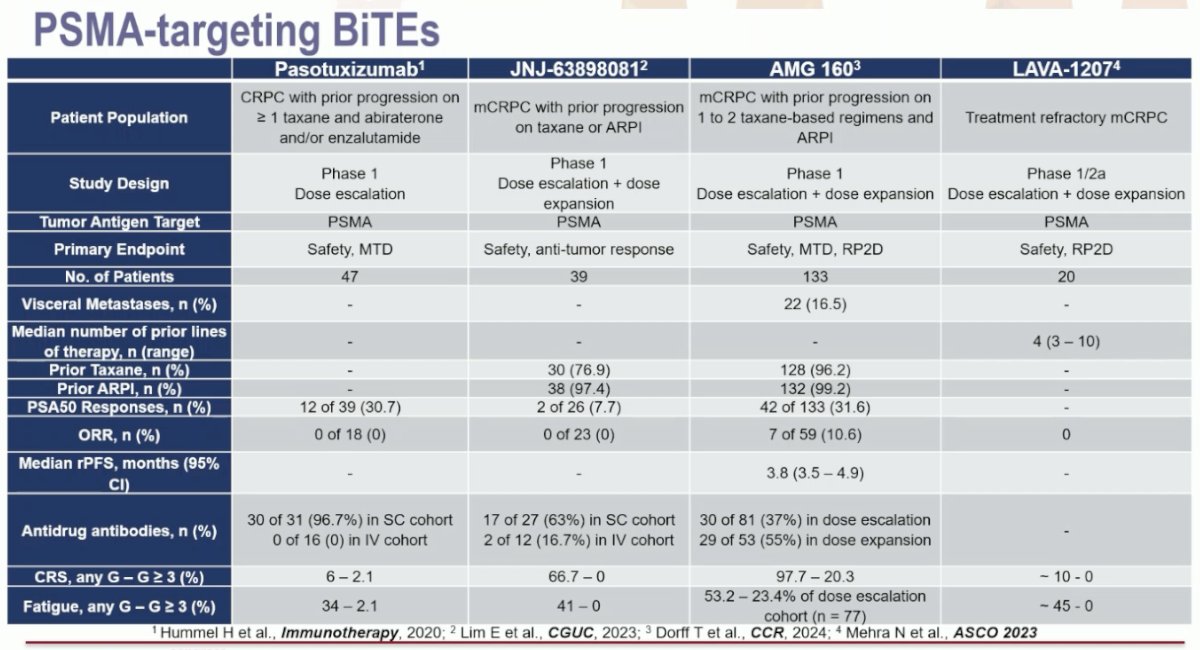
Xaluritamig (AMG 509) is a STEAP-1 targeting bi-specific T-cell engager that has been evaluated in mCRPC patients.
STEAP1 (six transmembrane epithelial antigen of the prostate 1) is associated with:
- Cancer cell proliferation
- Cancer invasion
- Epithelial-mesenchymal transition
STEAP1 staining was detected in 88% of evaluable matched mCRPC tissues. Patient-level analysis demonstrated that STEAP1 was expressed in 95% of patients compared to 68% for PSMA.4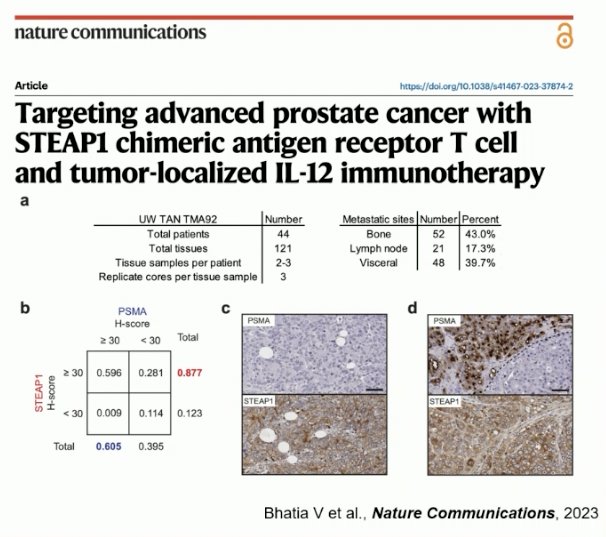
In a phase I study, the STEAP-1 targeting bi-specific T cell engager, Xaluritamig (AMG 509), was evaluated in heavily pre-treated mCRPC patients who had progressed on a prior androgen receptor pathway inhibitor (ARPI) and a taxane. 53% had visceral metastases, including 37% with liver metastases.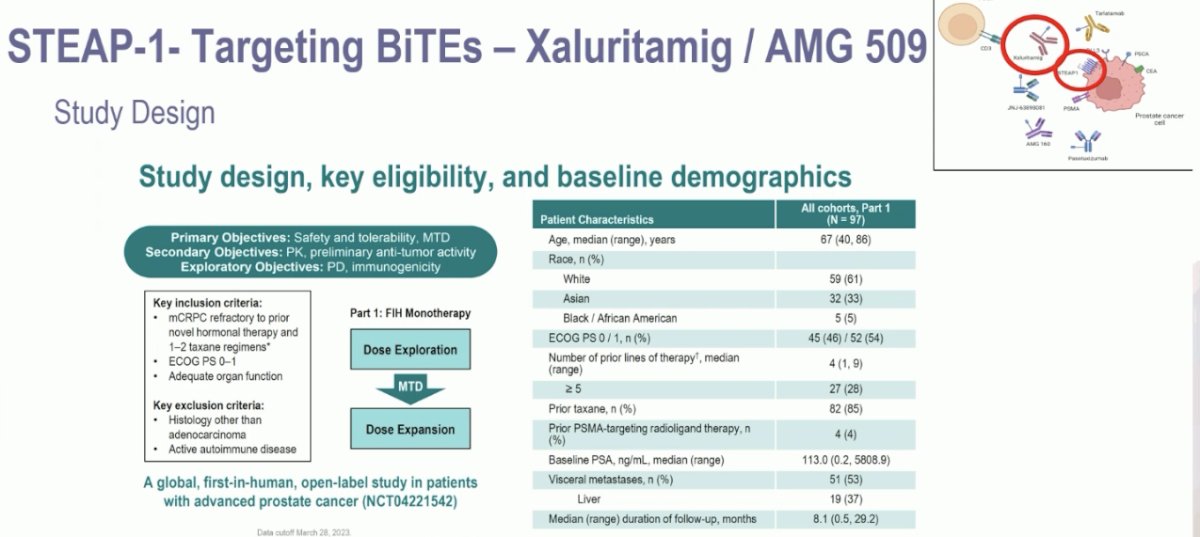
A PSA50 response was observed in 49% of patients, and a PSA90 response was observed in 30%.
From a safety standpoint, cytokine release syndrome was present in ~70% of patients. However, only 3% of patients discontinued this drug because of cytokine release syndrome. This drug will soon undergo evaluation in a phase III trial to be announced.
The DLL3-targeting bi-specific T cell engager, tarlatamab, was evaluated in a phase I trial of neuroendocrine prostate cancer. The results of this trial were presented at ASCO 2024. This trial included patients with de novo or treatment-emergent neuroendocrine prostate cancer with ≥2 alterations in TP53, RB1, and/or PTEN by immunohistochemistry or genomic analysis and progressed on ≥1 prior systemic treatment (platinum chemotherapy or androgen signaling inhibitor). In this trial, patients received tarlatamab 100 mg IV every two weeks with one-step dosing, as summarized below:
Efficacy outcomes were disappointing. The objective response rate in the overall cohort was 10.5%, and 22.2% in the DLL3+ cohort. The median duration of treatment was only 1.4 months overall (3.6 months in the DLL3+ cohort). The median duration of response was 7.3 months; 1 patient remains on treatment with an ongoing response at 25.8+ months. Dr. Beltran noted that only 56% of patients were DLL3+, and DLL3+ positivity was defined as ≥1% DLL3 tumor positivity by immunohistochemistry. As such, she argued that this may not be the ‘prime’ cohort of patients to benefit from such treatment.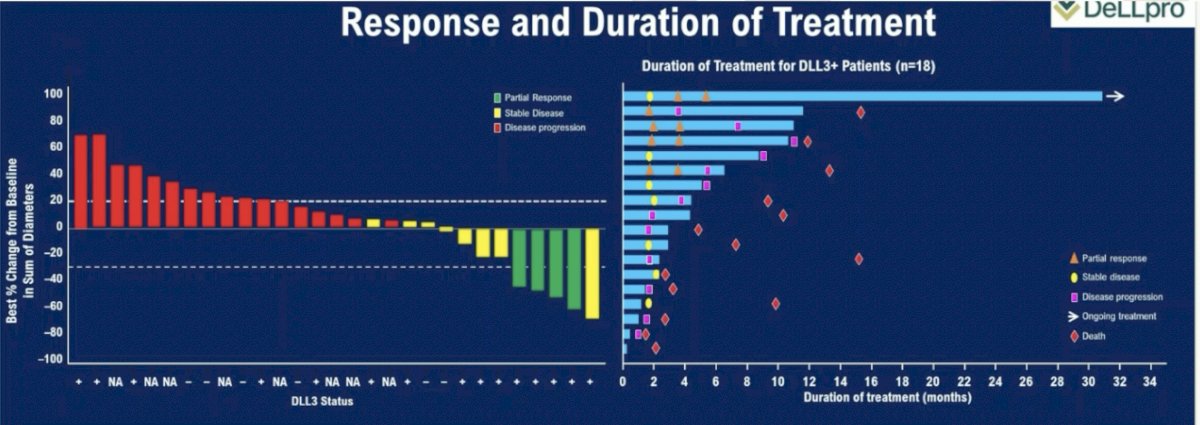
Cytokine release syndrome was present in 75% of patients; however, only 1 patient had such a grade ≥3 event. Immune effector cell-associated neurotoxicity was observed in 12.5% of patients (1 grade ≥3 event).
Summarized in the table below are the results of all studies of bi-specific T cell engagers in prostate cancer:
What about tri-specific T-cell engagers? These agents have a third binding domains that can be used for:
- Dual targeting of tumor-associated antigens
- Dual targeting of T cell receptors
- Fusion to human serum albumin, which extends its half-life, allowing for more even drug concentration and less frequent dosing
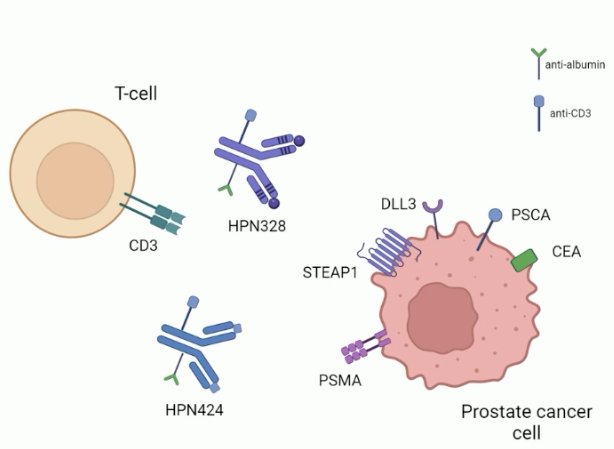
To date, two tri-specific T-cell engagers have been evaluated in prostate cancer:
- HPN424 (PSMA targeting)
- HPN328 (DLL3 targeting)

Overall, what we have learned so far is that these tri-specific T-cell engagers have manageable safety profiles and promising efficacy.
Summarized below are the single agent T cell engagers under development in prostate cancer, with novel targets including prostate stem cell antigen (PSCA) and Kallikrein-2 (KLK2).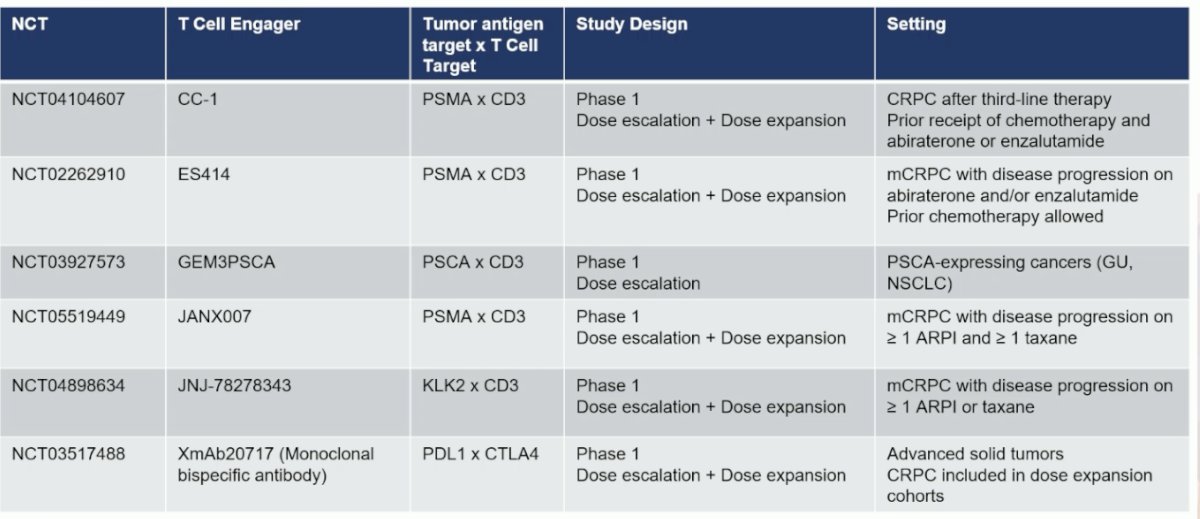
What have we learned so far about T cell engagers in prostate cancer? These are new therapies with a novel mechanism of action. Most have manageable safety profiles. There is subtle anti-tumor activity with most T cell engagers. Multiple challenges are faced by these agents, including:
- Immunogenicity: Anti-drug antibodies impact serum drug exposure
- Cytokine release syndrome: Most frequent adverse event, frequent with the first dose
How about improving the efficacy of T-cell engagers by combining them with other agents? Given the heterogeneous nature of prostate cancer, combination regimens with therapies that have an alternate mechanism of action may simultaneously target other components of the tumor microenvironment. In 2023, a phase II trial of pembrolizumab + an anti-CD3 x anti-HER2 bi-specific antibody T cell engager in mCRPC patients was published. This combination was demonstrated to be both active and safe.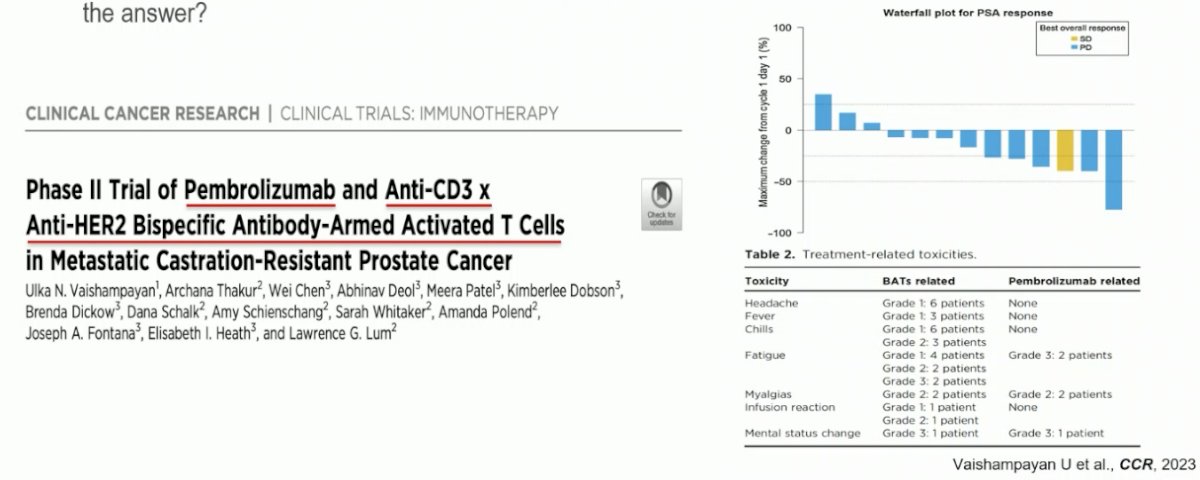
The combination of REGN5678 (co-stimulatory bi-specific PSMAxCD28 antibody) and cemiplimab (anti-PD-1) was tested in mCRPC patients. This combination was demonstrated to be safe with only 17% of patients experiencing a cytokine release syndrome (all grade 1 events).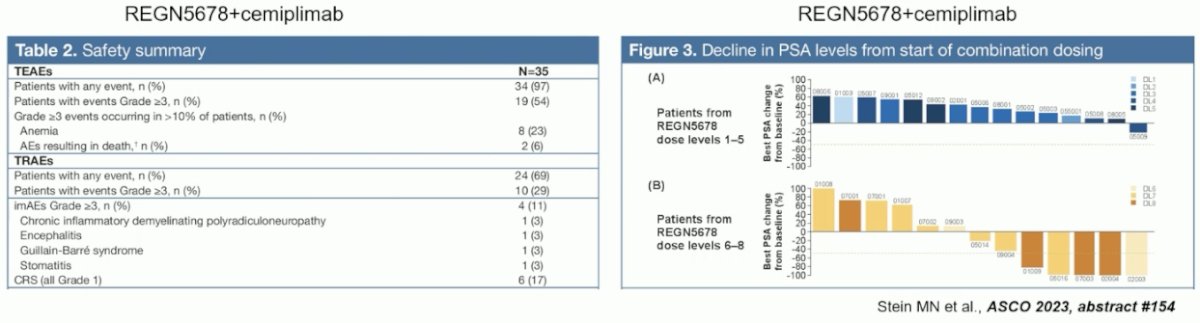
There are numerous other ongoing trials of combination T cell engager therapy + other agents that have non-overlapping targets:
What is the optimal timing for administering T cell engagers in the mCRPC disease course? Do we need to move them further up the treatment landscape? Xaluritamig/AMG 509 is being evaluated in mCRPC patients who have not progressed on taxanes or ARPIs.
Another important future consideration for the treatment with T cell engagers is biomarker selection. This was observed with improved tarlatamab responses in DLL3+ patients. Incorporating DLL-3-based imaging with a PET agent, such as 89Zr-DFO-DLL3-scFv, may be a potential solution in this setting.
Dr. Agarwal concluded as follows:
- T-cell engagers are emerging as a potential new tool in the therapeutic armamentarium for patients with advanced prostate cancer
- Various monotherapies and combinations have shown acceptable safety profiles
- The efficacy data are preliminary and modest for most of these agents
- Combinations with drugs that have non-overlapping mechanisms of action, moving to earlier lines of therapies, and biomarker selection may potentially improve the efficacy of these T-cell engagers
Presented by: Neeraj Agarwal, MD, Professor, Department of Medicine, Huntsman Cancer Institute, University of Utah, Salt Lake City, UT
Written by: Rashid Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
Related content: T-Cell Engagers in Prostate Cancer Treatment - Neeraj Agarwal
- Rathi N, McFarland TR, Nussenzveig R, Agarwal N, Swami U. Evolving Role of Immunotherapy in Metastatic Castration Refractory Prostate Cancer. Drugs. 2021; 81(2):191-206.
- Kantarjian H, Stein A, Gokbuget N, et al. Blinatumomab versus Chemotherapy for Advanced Acute Lymphoblastic Leukemia. N Engl J Med. 2017; 367(9):836-47.
- Ahn MJ, Cho BC, Felip E, et al. Tarlatamab for Patients with Previously Treated Small-Cell Lung Cancer. N Engl J Med. 2023; 389:2063-75.
- Bhatia V, Kamat NV, Pariva TE, et al. Targeting advanced prostate cancer with STEAP1 chimeric antigen receptor T cell and tumor-localized IL-12 immunotherapy. Nat Commun. 2023; 14(1):2041.
- Vaishampayan UN, Thakur A, Chen W, et al. Phase II Trial of Pembrolizumab and Anti-CD3 x Anti-HER2 Bispecific Antibody-Armed Activated T Cells in Metastatic Castration-Resistant Prostate Cancer. Clin Cancer Res. 2023; 29(1):122-133.


