(UroToday.com) The 2024 ESMO annual meeting included a session on radioligand theranostics in prostate cancer, featuring a presentation by Dr. Ken Herrmann discussing the status quo and new developments. PSMA can be visualized with PSMA-targeted radioligands that can be used for either PET imaging or therapeutic purposes. It is a transmembrane protein expressed on the surface of prostate cancer cells, with no appreciable circulation, and is highly expressed in prostate cancer cells within the tumor tissue (both primary and metastatic) in up to 80% of men with prostate cancer. Theranostics is a field that uses the same/similar targeting compound labeled with either a diagnostic or therapeutic radionuclide:

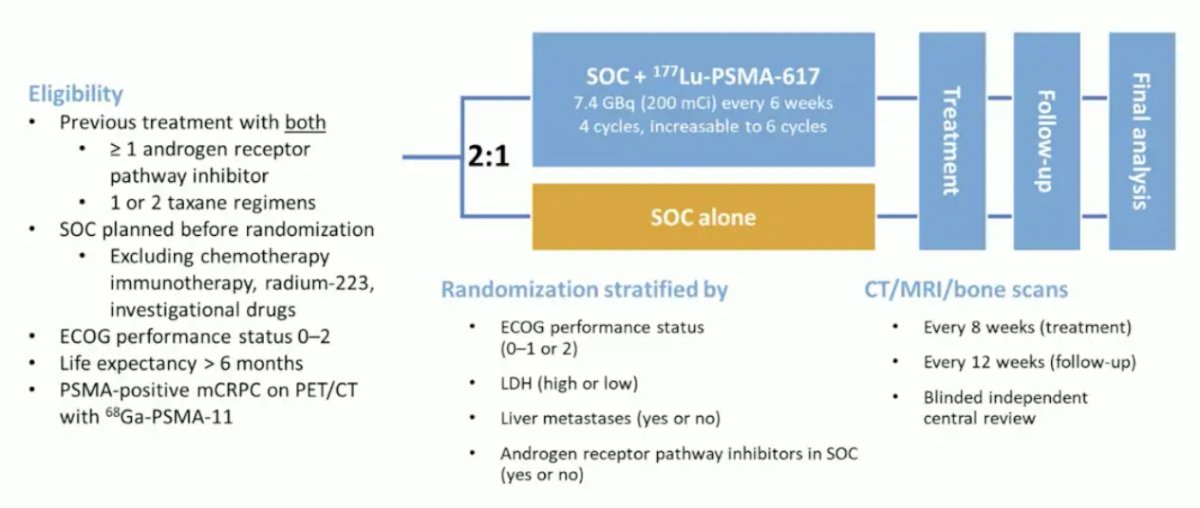
Dr. Herrmann then discussed several of the ground-breaking trials in the radionuclide therapy space, starting with the VISION trial.1 VISION was an international, randomized, open-label phase III study evaluating 177Lu-PSMA-617 in men with PSMA-positive mCRPC who had previously received treatment with a next-generation androgen receptor signaling inhibition (abiraterone, enzalutamide, etc) and one or two prior lines of taxane chemotherapy. Patients must have had an ECOG performance status of 0-2 and life expectancy of at least 6 months. Importantly, patients must have had PSMA-positive disease on the basis of a central review of 68Ga-PSMA-11 staging scans. PSMA positivity was defined as uptake greater in metastatic lesions than in the liver. Furthermore, patients could have no PSMA-negative metastatic lesions. Following enrollment, patients were randomized in a 2:1 fashion to receive either 177Lu-PSMA-617 (7.4 GBq every 6 weeks x 6 cycles) plus standard of care or standard of care alone. Standard of care treatments was at the discretion of the treating investigator; however, cytotoxic chemotherapy, immunotherapy, and radium-223 were explicitly excluded. The trial design for VISION is as follows:
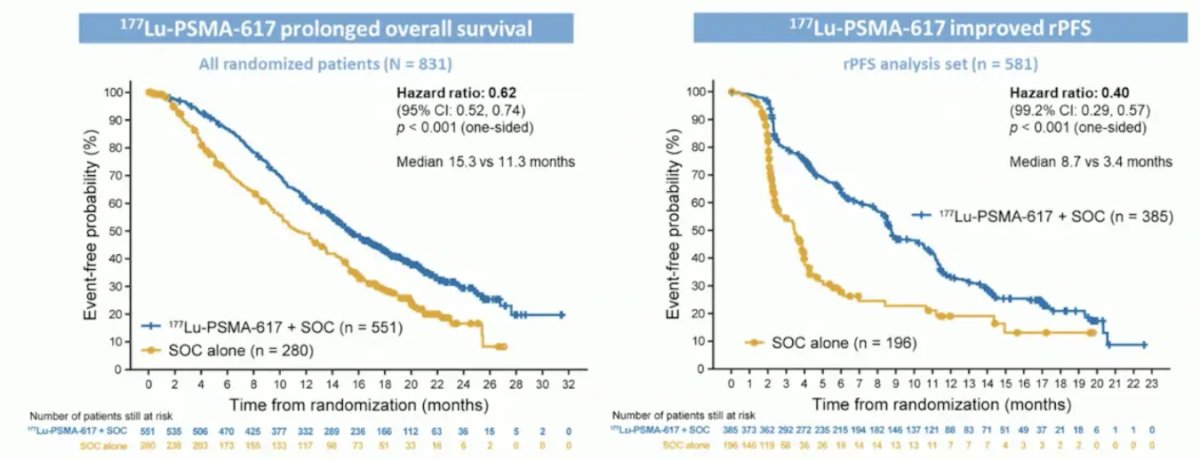
VISION enrolled 831 patients, and in keeping with the 2:1 randomization schema, 551 patients were allocated to 177Lu-PSMA-617 + standard of care and 280 were allocated to standard of care only. Over a median study follow-up of 20.9 months, treatment with 177Lu-PSMA-617 + standard of care significantly improved overall survival by a median of 4.0 months (median overall survival: 15.3 vs 11.3 months; HR 0.62, 95% CI 0.52 to 0.74; p < 0.001, one-sided), compared to standard of care alone, in the overall cohort of all randomized patients. With regards to the other primary endpoint of radiographic progression free survival, treatment with 177Lu-PSMA-617 + standard of care significantly improved rPFS by a median 5.3 months (median rPFS, 8.7 vs 3.4 months; HR 0.40, 99.2% CI 0.29 to 0.57; p < 0.001, one-sided):
Time to worsening in health-related quality of life and pain were also improved with 177Lu-PSMA-617 + standard of care. This included the FACT-P total score (HR 0.46, 95% CI 0.35 – 0.61) and BPI-SF pain intensity (HR 0.45, 95% CI 0.33 – 0.60):2

Dr. Herrmann then discussed the TheraP trial, which was the first randomized study to evaluate 177Lu-PSMA-617 vs cabazitaxel for men with mCRPC after docetaxel.3 In this open label, phase II trial, 200 men were randomized to either 177Lu-PSMA-617 or cabazitaxel. To screen into the study, all men had both 68Ga-PSMA-11 and 18F-FDG PET/CT and were required to have high PSMA-expression (at least one site with SUVmax ≥ 20) and no sites of FDG-positive/PSMA-negative disease. All patients had progressive disease with rising PSA ≥ 20 ng/mL after docetaxel and 91% had received prior enzalutamide or abiraterone. Overall, 200 patients were randomized 1:1 to 177Lu-PSMA-617 6-8 GBq every 6 weeks for up to 6 cycles of therapy or cabazitaxel 20 mg/m2 every 3 weeks for up to 10 cycles. Patients were stratified based on disease burden and prior anti-androgen therapy. The trial schema for TheraP is as follows:

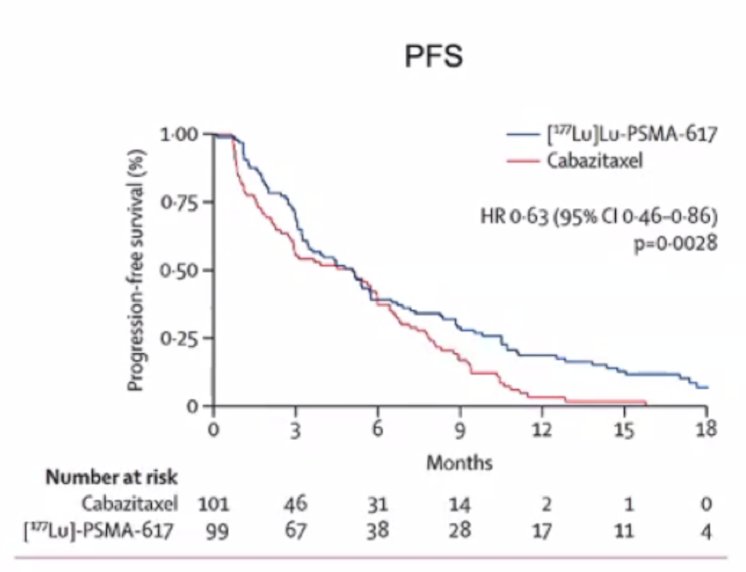
After a median follow up of 13 months, 177Lu-PSMA-617 significantly improved PSA-PFS compared with cabazitaxel (HR 0.63, 95% CI 0.46 to 0.86):
The EAU guidelines give a strong recommendation for “offering 177Lu-PSMA-617 to pre-treated mCRPC patients with one or more metastatic lesions, highly expressing PSMA (exceeding the uptake in the liver) on the diagnostic radiolabeled PSMA PET/CT scan.” The following table highlights radioligand therapy options for mCRPC options that are either approved or in development:

Dr. Herrmann then discussed the polling panel at APCCC 2024 in Lugano, Switzerland, specifically the question “For the majority of chemotherapy fit patients with PSMA imaging-positive mCRPC who meet relevant PET criteria for 177Lu-PSMA-617 therapy, who have received one line of an ARPI and no chemotherapy, what is your preferred treatment option assuming treatments are all readily available and there is no actionable molecular alteration?” Among asymptomatic patients, the prostate cancer experts voted that 70% would give docetaxel and 28% would give 177Lu-PSMA-617. If a patient was symptomatic, 82% would give docetaxel and 17% would give 177Lu-PSMA-617. If the question were slightly altered and the patient had already received chemotherapy, 96% of experts would treat with 177Lu-PSMA-617.
Dr. Herrmann then discussed the PSMAFore trial, which was presented at ESMO 2023, and assessed whether we can use 177Lu-PSMA-617 before chemotherapy. Eligible adults for PSMAfore had mCRPC, were candidates for ARPI change after one progression on prior ARPI, and had ≥1 PSMA positive lesions and no exclusionary PSMA negative lesions by 68Ga-PSMA-11 PET/CT. Candidates for PARP inhibition and patients with prior systemic radiotherapy (<6 months ago), immunotherapy (except sipuleucel-T), or chemotherapy (except [neo]adjuvant >12 months ago) were ineligible. Randomization was 1:1 to open-label 177Lu-PSMA-617 (7.4 GBq every 6 weeks for 6 cycles) or ARPI change (abiraterone or enzalutamide). Importantly, patients randomized to ARPI could crossover to 177Lu-PSMA-617 following centrally reviewed radiographic progression. The trial design for PSMAfore is as follows:
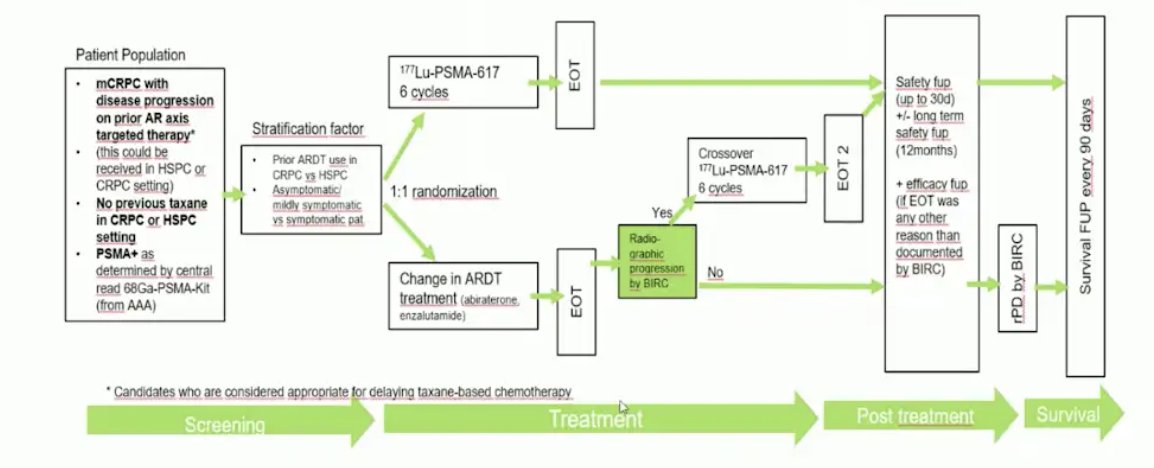
At the primary analysis (median follow-up, 7.3 months; n = 467), the primary endpoint of radiographic progression free survival was met (HR 0.41, 95% CI 0.29 to 0.56), which was similar at second interim analysis (HR 0.43, 95% CI 0.33 to 0.54):
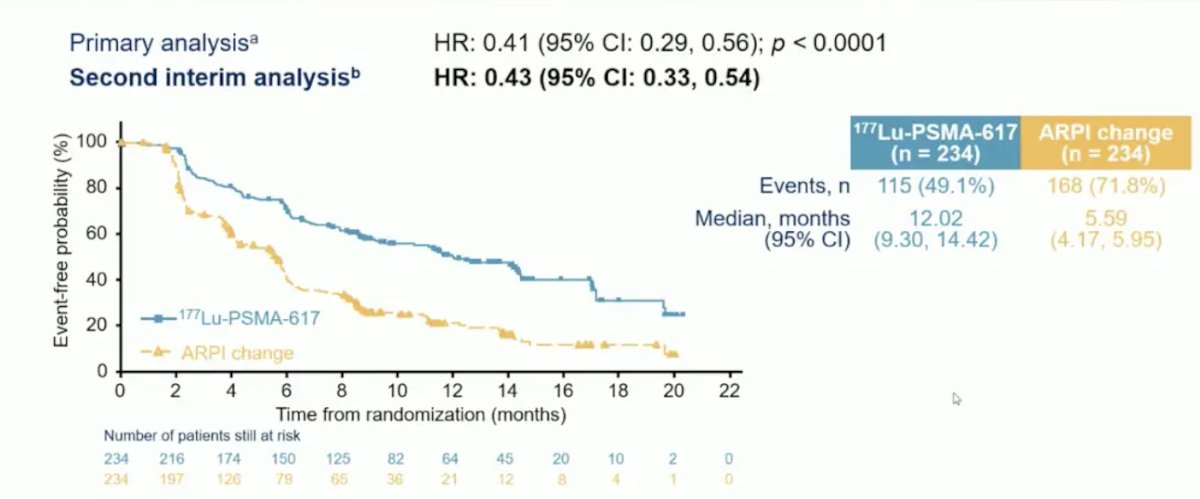
For overall survival, there was no difference between the groups (HR 0.98, 95% CI 0.75-1.28), most likely secondary to 57.3% of patients crossing over in the ARPI change group and crossing over occurring among 77.5% of eligible patients:

In a prespecified quality of life analysis, FACT-P total score was improved with 177Lu-PSMA-617 (HR 0.59, 95% CI 0.47-0.72), as was the EQ-5D-5L utility score (HR 0.61, 95% CI 0.50-0.76):

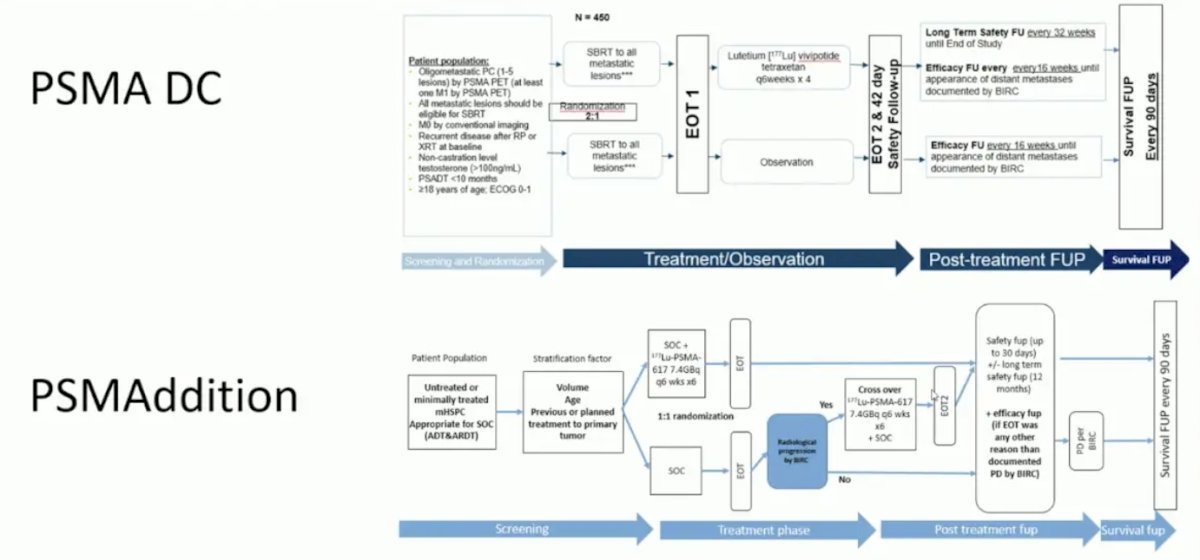
Dr. Herrmann then spent the remainder of his presentation discussing new developments, starting with PSMA DC in the oligometastatic setting and PSMAddition in the mHSPC space:
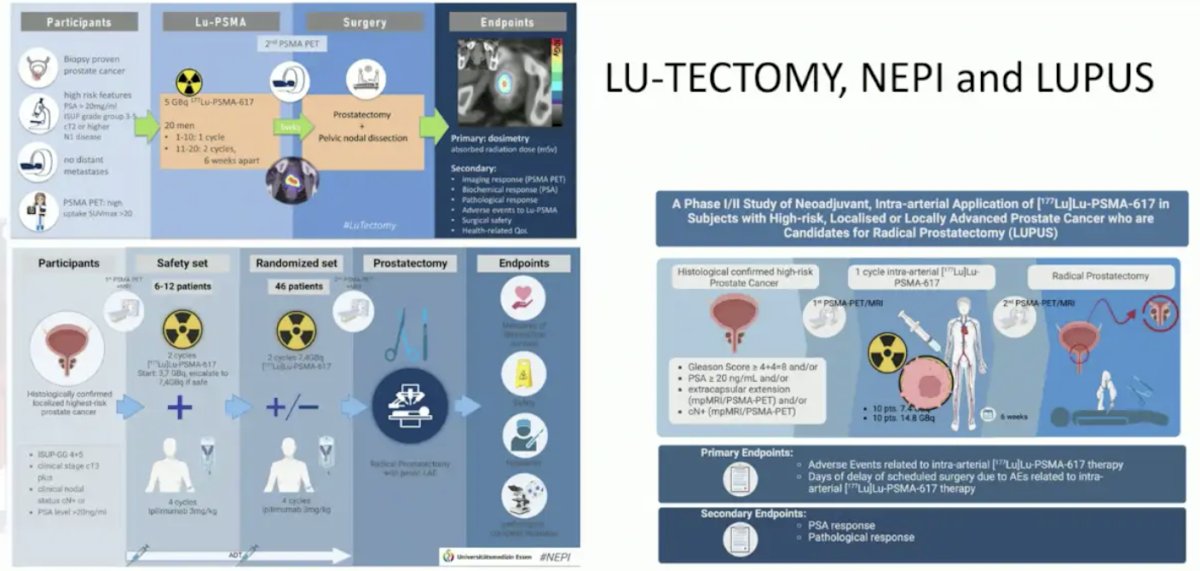
Similarly, Lu-Tectomy, NEPI, and LUPUS are assessing 177Lu-PSMA-617 in high risk localized prostate cancer:
The following highlights the current Lu-PSMA combination trials that are ongoing:

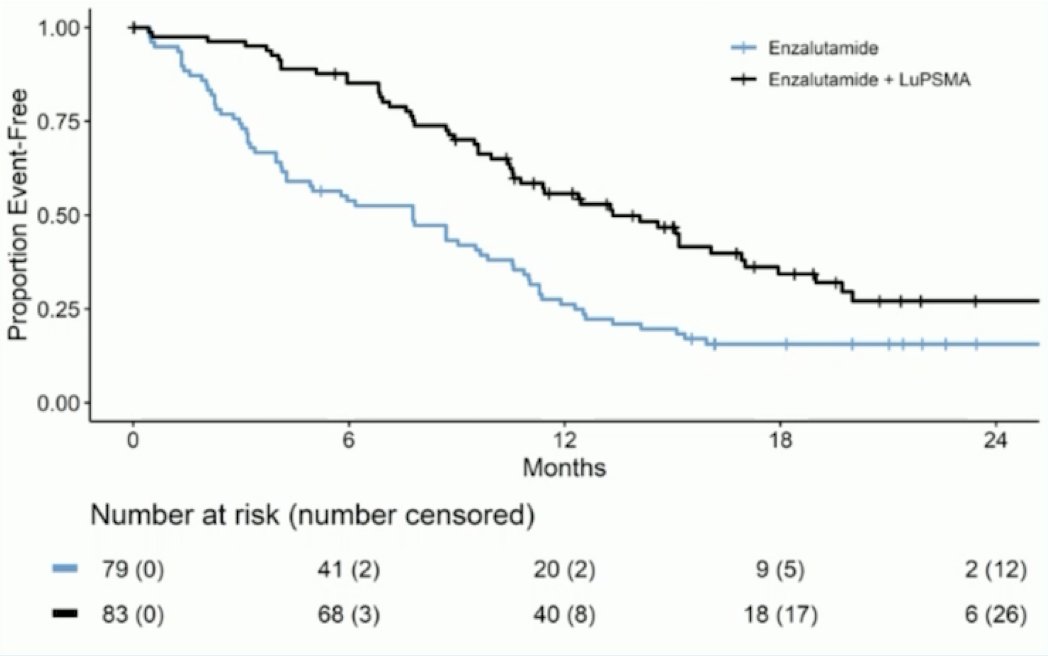
Two key combination trials include 177Lu-PSMA-617 + docetaxel + ADT vs docetaxel + ADT in the UpFrontPSMA trial (data to be presented at ESMO 2024) and the previously published Enza-P trial.4 Enza-P patients had mCRPC not previously treated with chemotherapy or androgen receptor pathway inhibitors (prior abiraterone and/or docetaxel for hormone-sensitive disease were allowed), 68Ga-PSMA-positive disease on PET, and at least 2 risk factors associated with early progression on enzalutamide. At the interim analysis, protocol treatment was ongoing in 48 patients, including 16 in the enzalutamide arm and 32 in the enzalutamide + LuPSMA arm. In the enzalutamide + LuPSMA arm, 81% of patients received four doses of LuPSMA. Over a median follow up of 20 months (IQR 18-21), PSA-PFS was longer with enzalutamide + LuPSMA vs enzalutamide-alone (median 13 vs 7.8 months; HR 0.43, 95% CI 0.29-0.63, p<0.001):
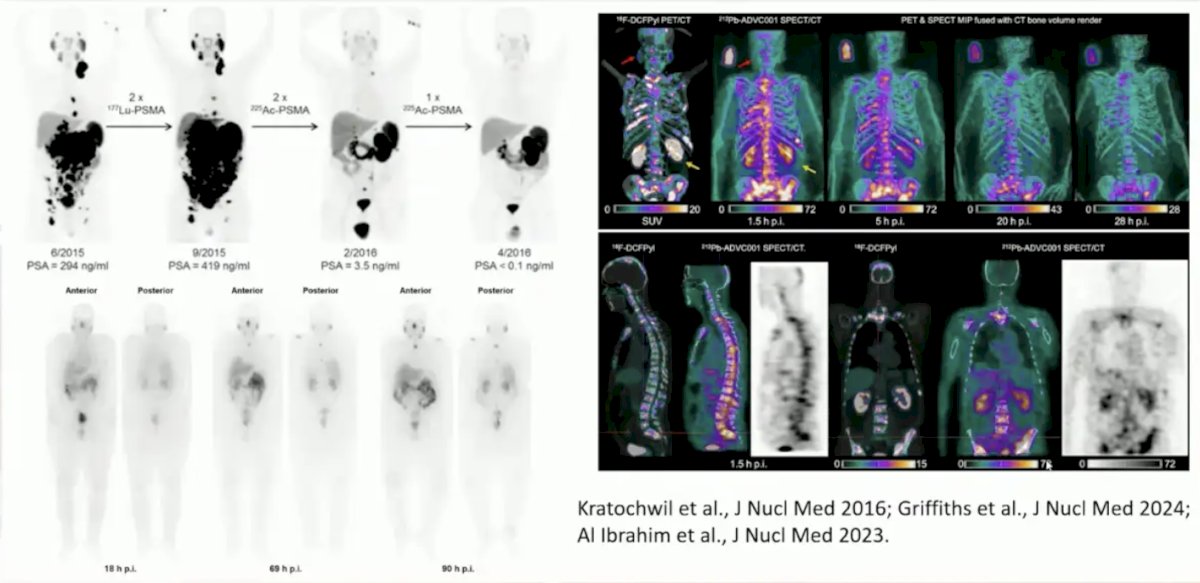
New Lu-PSMA radioligand versions, but in the same line of therapy include SPLASH, ECLIPSE, and PROSTACTGLOBAL. Additionally, there are newer radionuclides for PSMA, including 225Ac, 212Pb, and 161Tb:
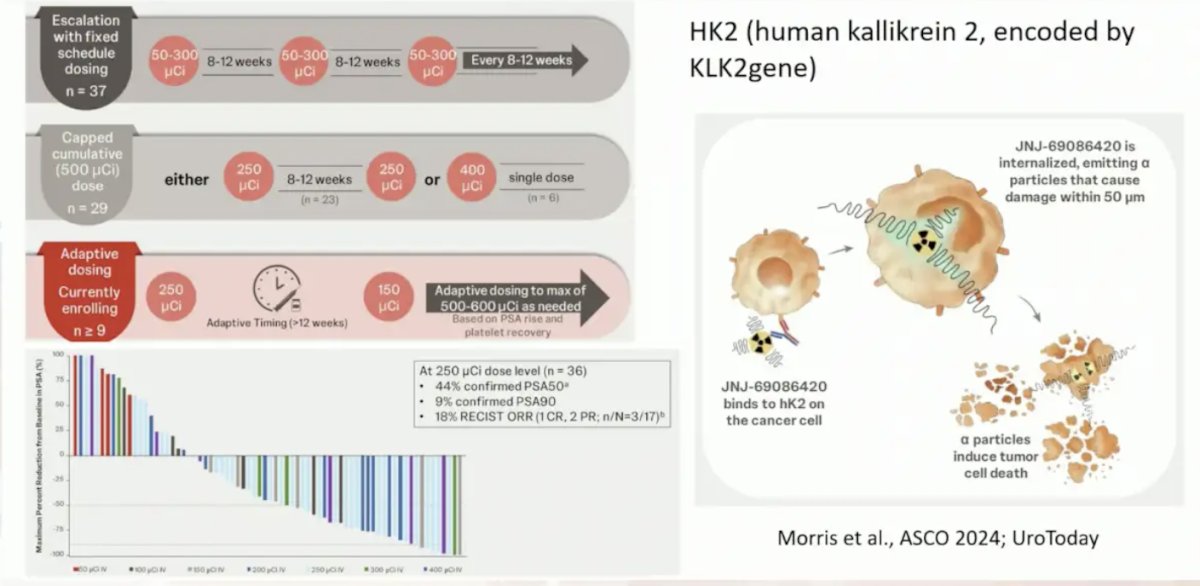
New targets other than PSMA, include human kallikrein 2 and GRP:
Nomograms have been developed for PSMA and FDG-PET as predictive and prognostic biomarkers.5 Buteau et al. previously showed that the hazard ratio for radiographic progression free survival for 177Lu-PSMA-617 versus cabazitaxel in men who had PSMA-PET SUVmean of at least 10 was 0.46 (95% CI 0.25–0.84), and was 0.85 (0.59–1.24) in men who had a PSMA-PET SUVmean of less than 10. Results were similar for PSA progression-free survival, with HRs for PSA progression-free survival of 0.45 (95% CI 0.25–0.80) for PSMA-PET SUVmean of at least 10 and 0.77 (0.53–1.12) for PSMA-PET SUVmean of less than 10.
Presented at ESMO 2024, Fendler and colleagues compared the prognostic value of PSMA-PET by PROMISE stage head-to-head with established clinical risk scores in a large prostate cancer dataset with overall survival follow-up. The PSMA-PET metrics by PROMISE are as follows:
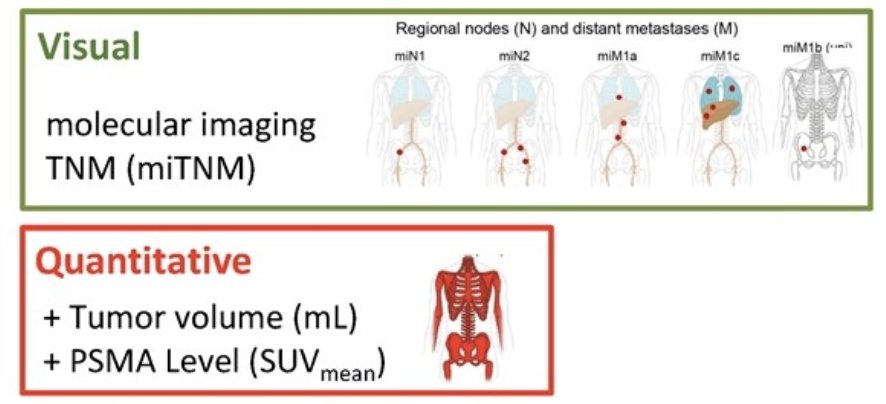
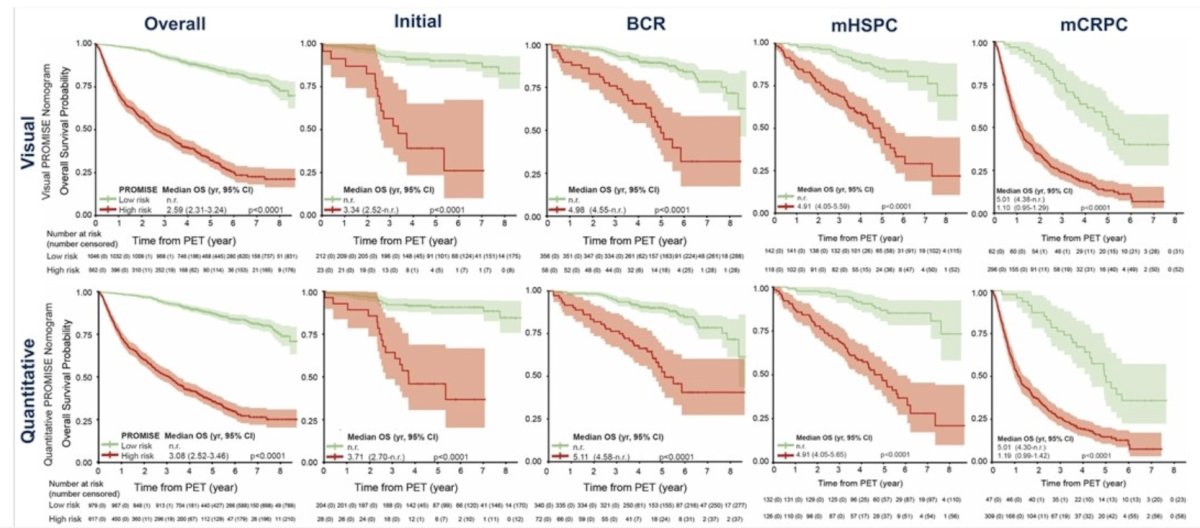
The investigators analyzed 2,414 patients (1,110 development, 502 internal and 802 external validation) with 901 (37.3%) deaths across all disease stages (median follow-up 52.9 months (IQR 33.9-79.0). Predictors in the quantitative PSMA-PET by PROMISE nomogram were locoregional lymph node metastases (miN2), distant metastases (miM1a, miM1b pattern, miM1c), tumor volume, and tumor SUVmean. The visual PSMA-PET by PROMISE nomogram includes distant metastases and total tumor lesion count. C-indices in the internal and external validation cohorts were 0.80 and 0.77 (quantitative) or 0.78 and 0.77 (visual), respectively. The following shows overall survival curves for all patients, as well as for biochemical recurrence, metastatic hormone sensitive prostate cancer, and metastatic castration resistant prostate cancer subgroups:
Dr. Herrmann concluded his presentation by discussing the status quo and new developments in radioligand theranostics with the following take-home points:
- Radioligand therapy is gaining acceptance and is on the trajectory to be another pillar of prostate cancer therapy
- VISION and PSMAfore set the stage
- New concepts of radioligand therapy include:
- Moving into earlier lines (PSMAddition, PSMA-DC, Lu-Tectomy)
- New versions of Lu-PSMA (SPLASH, ECLIPSE, PROSTACTGLOBAL)
- Combination therapy approaches (Enza-P, UpFrontPSMA)
- New radionuclines (225Ac, 212Pb, 161Tb)
- Novel prostate cancer targets (HK2, GRP)
- Power of imaging (Prognostication, Prediction)
Presented by: Ken Herrmann, MD, University of Essen, Essen, Germany
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
Related content: Advancements in Lutetium-PSMA Therapy for Prostate Cancer: Clinical Trials and Future Directions - Ken Herrmann
References:
- Sartor O, de Bono J, Chi KN et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021 Sep 16;385(12):1091-1103.
- Fizazi K, Herrmann K, Krause BJ, et al. Health-related quality of life and pain outcomes with [177Lu]Lu-PSMA-617 plus standard of care versus standard of care in patients with metastatic castration-resistant prostate cancer (VISION): A multicentre, open-label, randomized, phase 3 trial. Lancet Oncol. 2023 Jun;24(6):597-610.
- Hofman MS, Emmett L, Sandhu S, et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomized, open-label, phase 2 trial. Lancet. 2021 Feb 27;397(10276):797-804.
- Emmett L, Subramaniam S, Crumbaker M, et a. [177Lu]Lu-PSMA-617 plus enzalutamide in patients with metastatic castration-resistant prostate cancer (ENZA-p): An open-label, multicentre, randomized, phase 2 trial. Lancet Oncol. 2024 May;25(5):563-571.
- Buteau JP, Martin AJ, Emmett L, et al. PSMA and FDG-PET as predictive and prognostic biomarkers in patients given [177Lu]Lu-PSMA-617 versus cabazitaxel for metastatic castration-resistant prostate cancer (TheraP): A biomarker analysis from a randomized, open-label, phase 2 trial. Lancet Oncol. 2022 Nov;23(11):1389-1397.