(UroToday.com) The 2024 ESMO annual meeting included a session on expanding bladder preservation by optimal use of systemic therapy and biomarkers, featuring a presentation by Dr. James Catto discussing the role of the urologist in bladder preservation strategies.
To outline his talk, Dr. Catto used ‘The 5 S’s’:
- Selection – help find the appropriate cases
- Staging – response to neoadjuvant therapy
- Surveillance – for recurrence
- Salvage – if needed
- Support
The key question for selection is how many invasive bladder cancer patients are suitable for bladder preservation? Tumor features may make up 10-15% of patient selection, with less favorable features being: (i) hydronephrosis (relative), (ii) tumor in a diverticulum, (iii) widespread CIS, (iv) multiple tumors, and (v) lower urinary tract symptoms. Patient features may contribute 1-5% of patient selection including features such as: (i) ulcerative colitis, inflammatory bowel disease, (ii) previous pelvic radiotherapy, (iii) small bowel pathology, and (iv) hip replacements. Dr. Catto notes that there are several relatively firm reasons to exclude patients from bladder sparing:
- Widespread CIS
- Upper tract, urethral, or prostatic urothelial carcinoma
- Untreated hydronephrosis
- Invasive malignancy in the previous 5 years
Ideal patients, taken from the Toronto-centered multi-institutional series, include:1
- Solitary tumors less than 7 cm
- No or unilateral hydronephrosis
- No extensive or multifocal CIS
However, Dr. Catto notes that based on the aforementioned criteria, only 29% of patients underwent radical cystectomy fit the criteria for being candidates for bladder sparing.1 However, with the use of mpMRI are we able to better select patients?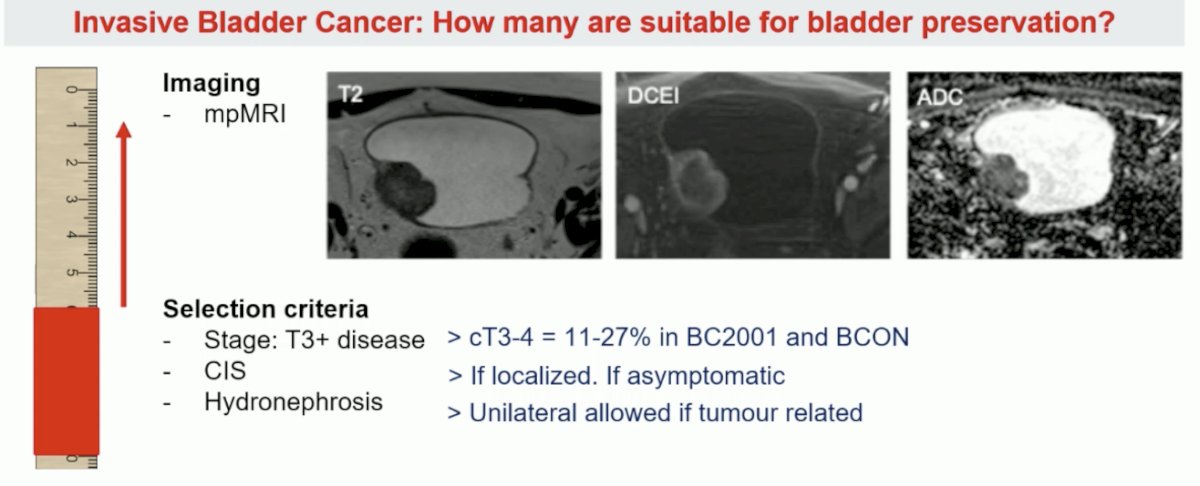
A key aspect of selecting patients for bladder-sparing treatment options is maximal TURBT as part of trimodality therapy. To support this, Dr. Catto notes that a quality/aggressive TURBT and salvage cystectomy (for the 10-15% that require it) is paramount to the success of trimodality therapy. Massachusetts General Hospital has excellent long-term results with trimodality therapy that are comparable to radical cystectomy: among cT2 disease, 5-year DSS is 74% and 10-year DSS is 66%.1 Among patients treated in a contemporary setting (2005-2013), salvage cystectomy rates are only 15%.1 The importance of TURBT extent is highlighted by the following disease-specific and overall survival curves stratified by TURBT extent: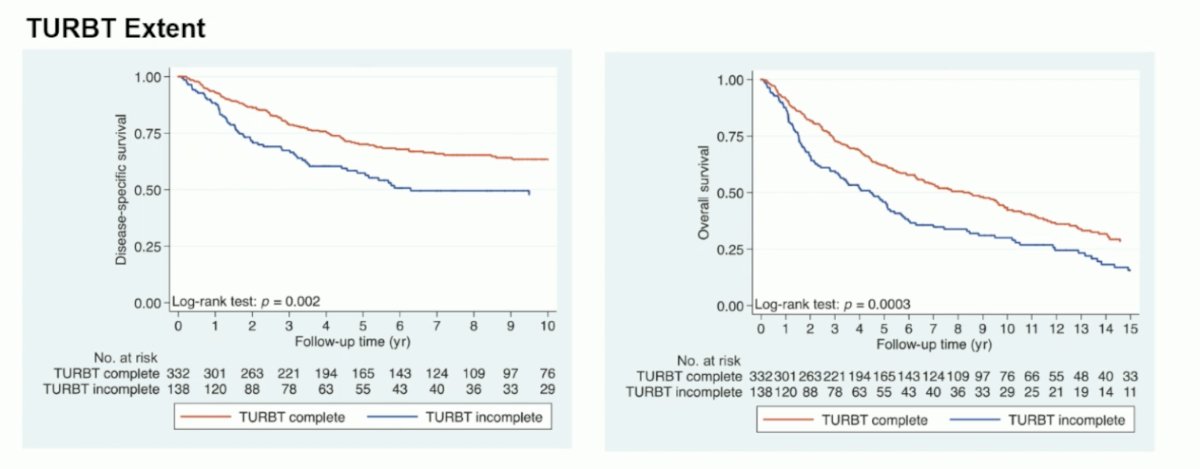
Another key aspect of the selection of patients is tumor sensitivity. Using molecular subtypes, Seiler et al. showed that luminal tumors had the best overall survival with and without neoadjuvant chemotherapy.3 Claudin-low tumors were associated with poor overall survival irrespective of the treatment regimen, and basal tumors showed the most improvement in overall survival with neoadjuvant chemotherapy compared with surgery alone.
Dr. Catto then discussed the novel BladderPath trial examining the role of MRI for bladder cancer staging. Based on the potential for muscle-invasive disease (assessed on a Likert scale at flexible cystoscopy), patients were enrolled and were randomized to standard of care with TURBT assessment (Pathway 1) or MRI-based assessment (Pathway 2) with tumor biopsy. Patients who were felt, based on cystoscopy, to have probable NMIBC all underwent TURBT. The first results of BladderPath were presented by Dr. Nick James at ESMO 2022. There were 143 patients randomized between May 2018 and December 2021. Based on cystoscopic assessment, 47.9% of patients were assessed to have probable NMIBC while 52.1% were assessed to have possible muscle-invasive bladder cancer. During the initial feasibility phase, 37 of 39 (95%, 95% CI 83-99%) patients with muscle-invasive bladder cancer followed the correct pathway, exceeding the target of 80%. Subsequently, during the efficacy phase, the median time to correct muscle-invasive bladder cancer treatment was 98 (95% CI 72-174) days for patients in the traditional Pathway 1 and 53 (95% CI 20-89) days for patients in Pathway 2 (p = 0.0046).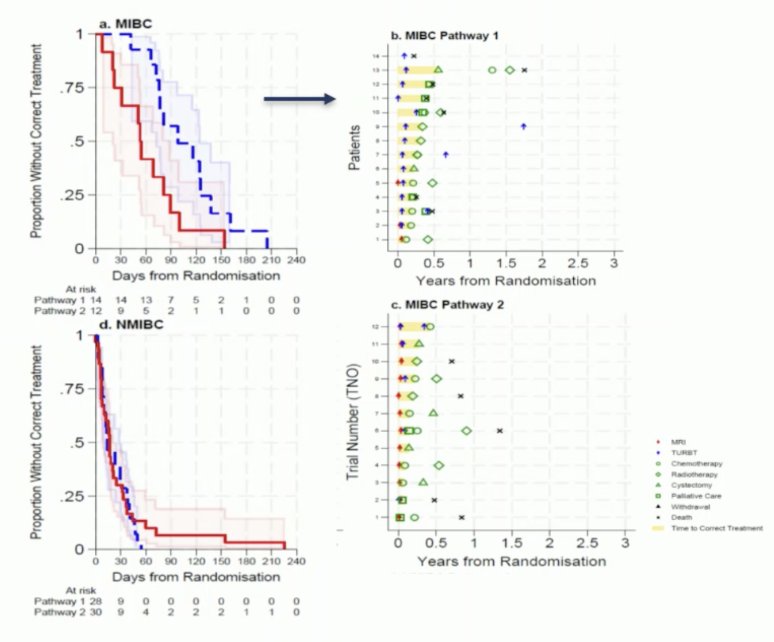
Dr. Catto then discussed staging, specifically response to neoadjuvant therapy, which includes cystoscopy +/- biopsy and cross-sectional imaging. Assessing response is crucial as it has been shown to portend outcomes, whether in RCTs or observation/single-center studies: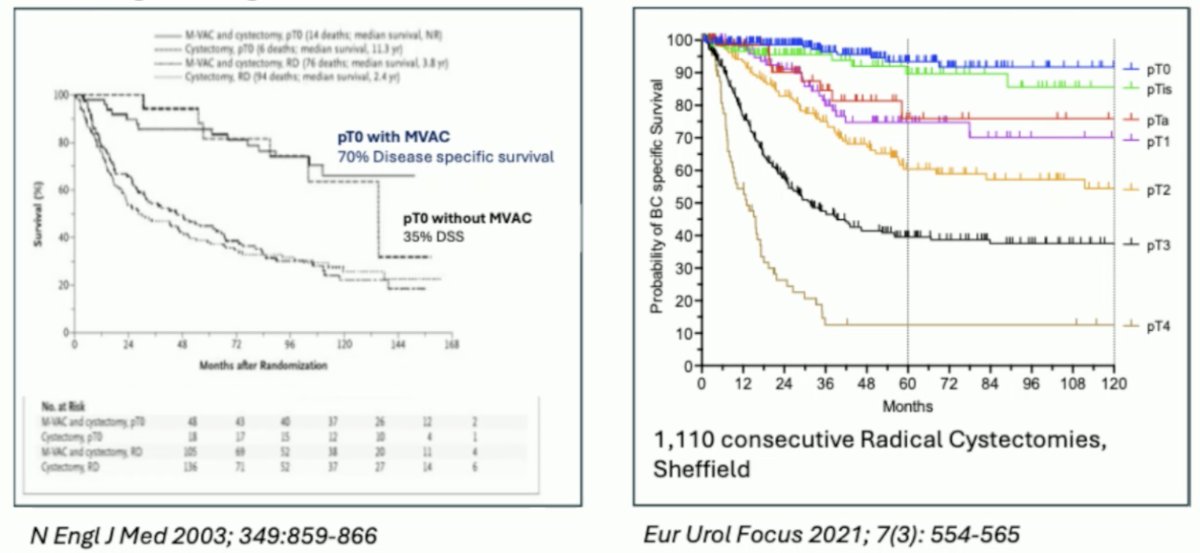
Unfortunately, it is difficult to measure response visually. Zibelman and colleagues previously performed a prospective trial assessing cystoscopy and systematic bladder tissue sampling in predicting pT0 bladder cancer at Fox Chase Cancer Center [4]. This was a single-arm study to evaluate the reliability of Systematic Endoscopic Evaluation (SEE) in predicting pT0 urothelial carcinoma. Overall, 61 patients underwent neoadjuvant chemotherapy, SEE, and radical cystectomy. On SEE, 31 (50.8%) demonstrated no visual or biopsy-based evidence of disease (SEE T0). However, among these patients 16/31 (51.6%) harbored residual disease (>pT0), and 8/31 (25.8%) had residual >= pT2 disease at the time of cystectomy. The negative predictive value of SEE predicting pT0 bladder cancer was 48.4% (95% CI 30.2-66.9), which was below the pre-specified hypothesis, thus the trial was stopped for futility.
Dr. Catto notes that with changing times come potentially better treatments, including new neoadjuvant treatment options: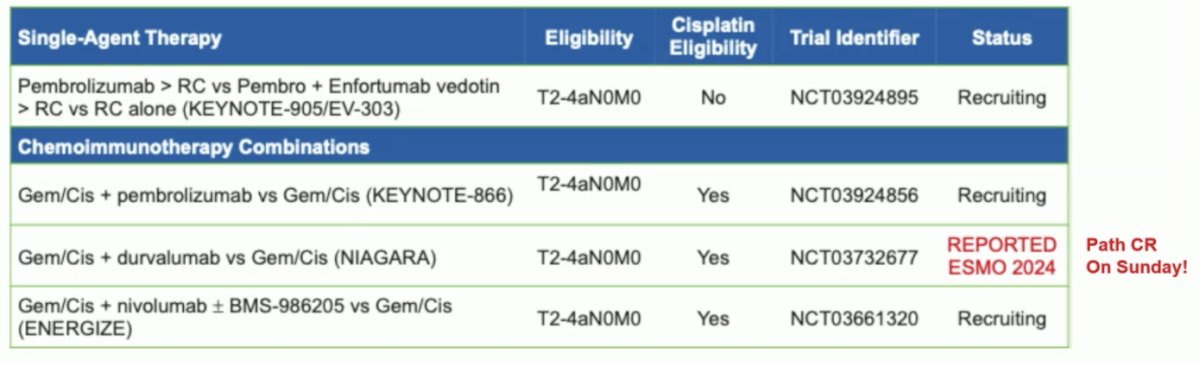
Surveillance and salvage are both crucial to a successful bladder-sparing program, and the following highlights Dr. Catto’s rigorous cystoscopy and CT imaging follow-up protocol: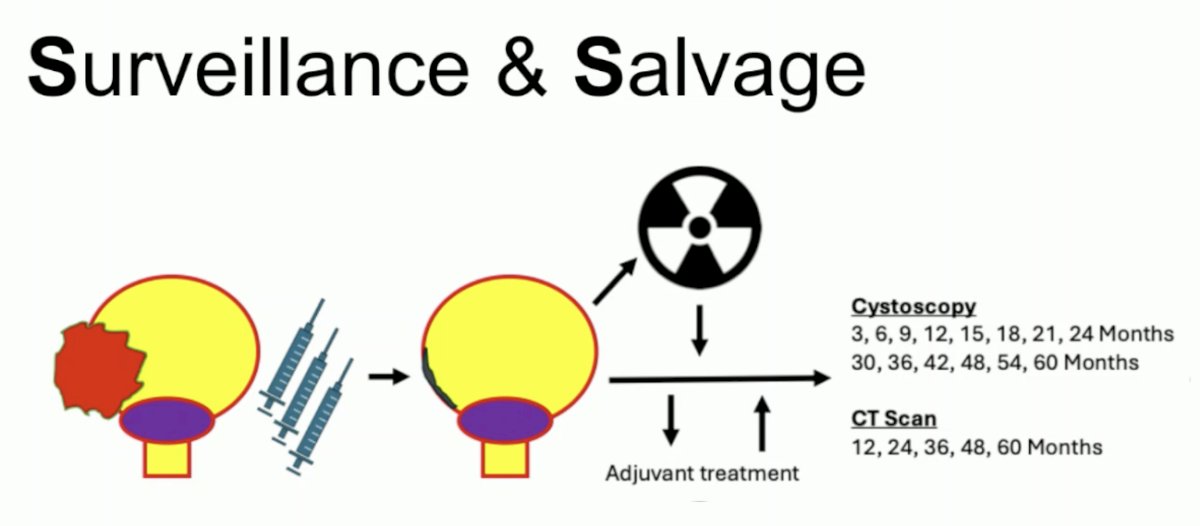
A 2021 systematic review assessed salvage outcomes among patients undergoing trimodal therapy, noting that among 73 studies and 9,110 patients:5
- Mean follow-up was 61.1 months (range: 12-144)
- Pooled rate of salvage cystectomy: 19.2%
- Overall complication rate: 67-72%
- 30-day mortality rate: 0-8.8%
- Pooled 5-year disease-free survival: 54.3%
- Pooled 10-year disease-free survival: 45.6%
Dr. Necchi and colleagues have also assessed surveillance of response to neoadjuvant pembrolizumab using mpMRI and the VI-RADS scoring system.6 In the PURE-01 trial, 110 patients underwent centrally reviewed scans (n = 220 mpMRI). Both pre- and post-pembrolizumab VI-RADS 0-3 scores were the only significant covariates that predicted the ypT≤1N0 endpoint in multivariable analyses, and the strongest effect was seen with post-pembrolizumab VI-RADS 0-3 predicting the ypT≤1N0 response (p < 0.001):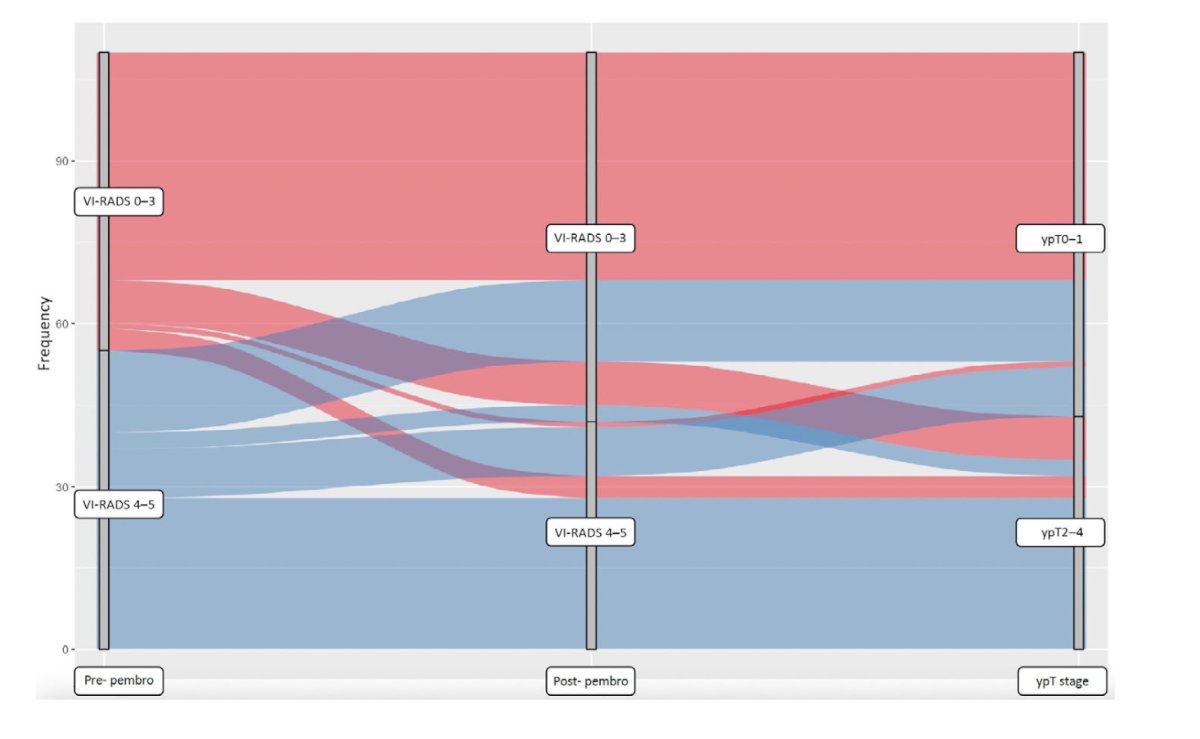
The area under the curve for this model was 0.90. Post-pembrolizumab VI-RADS 0-3 also predicted a longer event-free survival (p < 0.001) and overall survival (p = 0.044).
Finally, for surveillance, Dr. Catto discussed the impact of ctDNA, which has shown promise as a biomarker for response to neoadjuvant chemotherapy and oncological outcomes: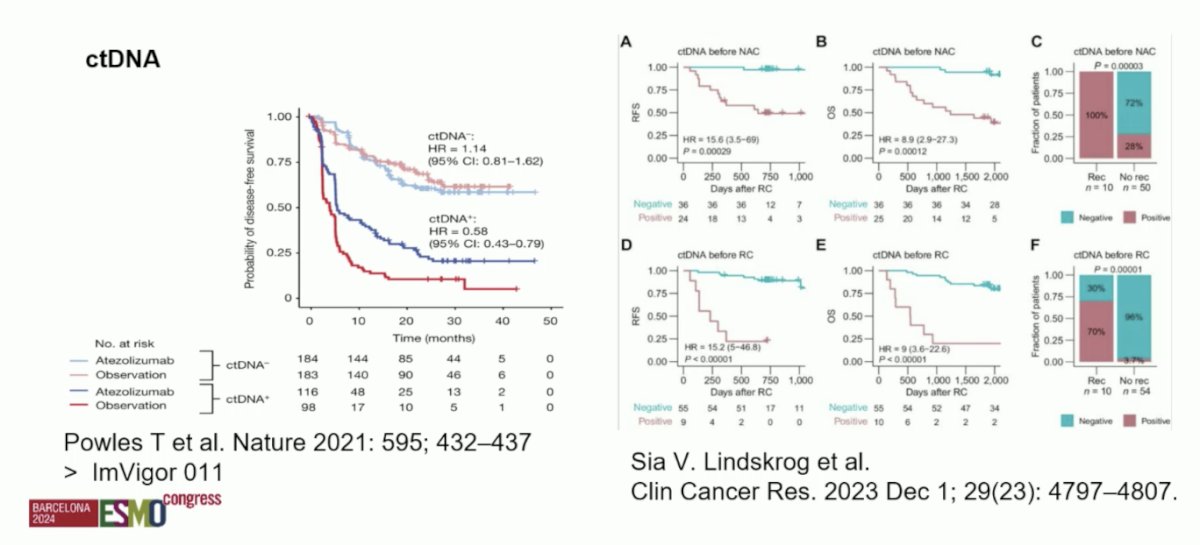
Dr. Catto concluded his presentation discussing the role of the urologist in bladder preservation strategies by again summarizing ‘The 5 S’s’, but also adding a sixth S: Staying up to date with the literature, particularly as a urologist, given the important collaboration with radiation oncology and medical oncology in bladder sparing management.
Presented by: James Catto, PhD, Professor, University of Sheffield, Sheffield, UK
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
- Zlotta AR, Ballas LK, Niemierko A, et al. Radical cystectomy versus trimodality therapy for muscle-invasive bladder cancer: A multi-institutional propensity score matched and weighted analysis. Lancet Oncol. 2023 Jun;24(6):669-681.
- Giacalone NJ, Shipley WU, Clayman RH, et al. Long-term outcomes after bladder-preserving tri-modality therapy for patients with muscle-invasive bladder cancer: An updated analysis of the Massachusetts General Hospital experience. Eur Urol. 2017;71:952-960.
- Seiler R, Al Deen Ashab H, Erho N, et al. Impact of molecular subtypes in muscle-invasive bladder cancer on predicting response and survival after neoadjuvant chemotherapy. Eur Urol. 2017 Oct;72(4):544-554.
- Zibelman M, Asghar AM, Parker DC, et al. Cystoscopy and systematic bladder tissue sampling in predicting pT0 Bladder Cancer: A Prospective Trial. J Urol. 2021 Jun;205(6):1605-1611.
- Schuettfort VM, Pradere B, Quhal F, et al. Incidence and outcome of salvage cystectomy after bladder sparing therapy for muscle-invasive bladder cancer: A systematic review and meta-analysis. World J Urol. 2021 Jun;39(6):1757-1768.
- Necchi A, Basile G, Gibb EA, et al. Vesical Imaging-Reporting and Data System use predicting the outcome of neoadjuvant pembrolizumab in muscle-invasive bladder cancer. BJU Int. 2024 Feb;133(2):214-222.


