(UroToday.com) The 2024 ESMO annual meeting included a session on addressing uncertainties in the management of urothelial and renal cell carcinomas, featuring a presentation by Dr. Begona Perez-Valderrama discussing how to build on standard therapies by assessing novel agents and promising combinations. Since 2010, there have been numerous FDA and EMA approvals of new agents in renal cell carcinoma, prostate cancer, and urothelial carcinoma:

Moreover, Dr. Perez-Valderrama notes that there are currently 351 active trials in renal cell carcinoma, 188 active trials in prostate cancer, and 929 active trials in urothelial carcinoma.
Dr. Perez-Valderrama then discussed several ongoing clinical trials assessing novel agents and promising combinations in RCC and urothelial carcinoma. Starting with RCC, she notes that the metastatic disease landscape has become quite busy over the last several years:
However, given the resistance to immunotherapy and antiangiogenic treatments, there is a need for additional novel therapies and combination regimens:
Arguably the most important novel agent, and perhaps the one that may change the landscape of RCC therapy, is belzutifan. Presented at ESMO 2024 by Dr. Brian Rini, LITESPARK-005 is a phase 3 trial randomizing previously treated patients 1:1 to belzutifan 120 mg or everolimus 10 mg QD until progression or unacceptable adverse events. Progression-free survival per RECIST 1.1 by central review and overall survival were the dual primary endpoints. Objective response rate per RECIST 1.1 by central review was a key secondary endpoint. Duration of response and safety were secondary endpoints. The trial design for LITESPARK-005 is as follows:
The progression-free survival benefit was maintained with belzutifan vs everolimus (median 5.6 months versus 5.6 months; HR 0.75; 95% CI 0.63–0.88), with an estimated progression-free survival rate at 12 months (33.7% vs 17.6%) and at 24 months (17.5% vs 4.1%) favoring belzutifan:
Progression-free survival across the subgroups generally showed an excellent benefit for belzutifan versus everolimus:
Median overall survival was 21.4 months with belzutifan versus 18.2 months with everolimus (HR 0.92; 95% CI 0.77–1.10; p = 0.18). Estimated overall survival rate was 67.9% vs 65.8% at 12 months and 45.2% vs 41.2% at 24 months:
Objective response rate was consistent with prior reports (22.7% vs 3.5%), and median duration of response was 19.3 months (range: 1.9+–40.1+) versus 13.7 months (range: 3.8–29.5+):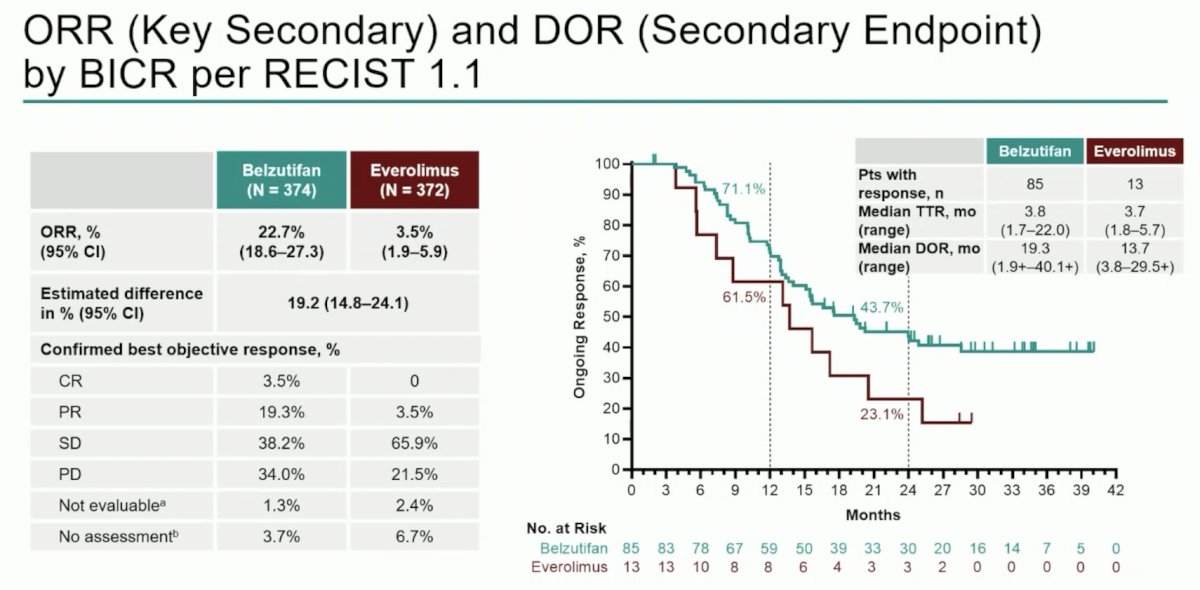
One novel combination is belzutifan with cabozantinib, which was tested in the phase 2 LITESPARK-003 trial.1 Among 52 patients in cohort 2, 16 (30.8%, 95% CI 18.7-45.1) of 52 patients had a confirmed objective response, including one (2%) who had a complete response and 15 (29%) who had partial responses. Belzutifan in combination with lenvatinib was assessed in the KEYMAKER-U038 (n = 30), noting an objective response rate of 50% (95% CI 29-71), but with a grade 3-4 adverse event incidence of 50%. There are several ongoing trials incorporating belzutifan, including LITESPARK-011, LITESPARK-012, and LITESPARK-024:

Another alternative proangiogenic pathway inhibitor in RCC is XL-092, which is being tested in the STELLAR-001 and STELLAR-002 trials:
177Lu-girentuximab is a radioligand therapy targeting CAIX, which has previously noted a stable disease rate of 74% in 23 heavily pre-treated clear cell RCC patients, but with a dose-limiting toxicity of myelotoxicity. There are several ongoing trials incorporating 177Lu-girentuximab:
Dr. Perez-Valderrama then discussed novel agents and promising combinations in urothelial carcinoma. The current landscape for metastatic urothelial carcinoma is highlighted below: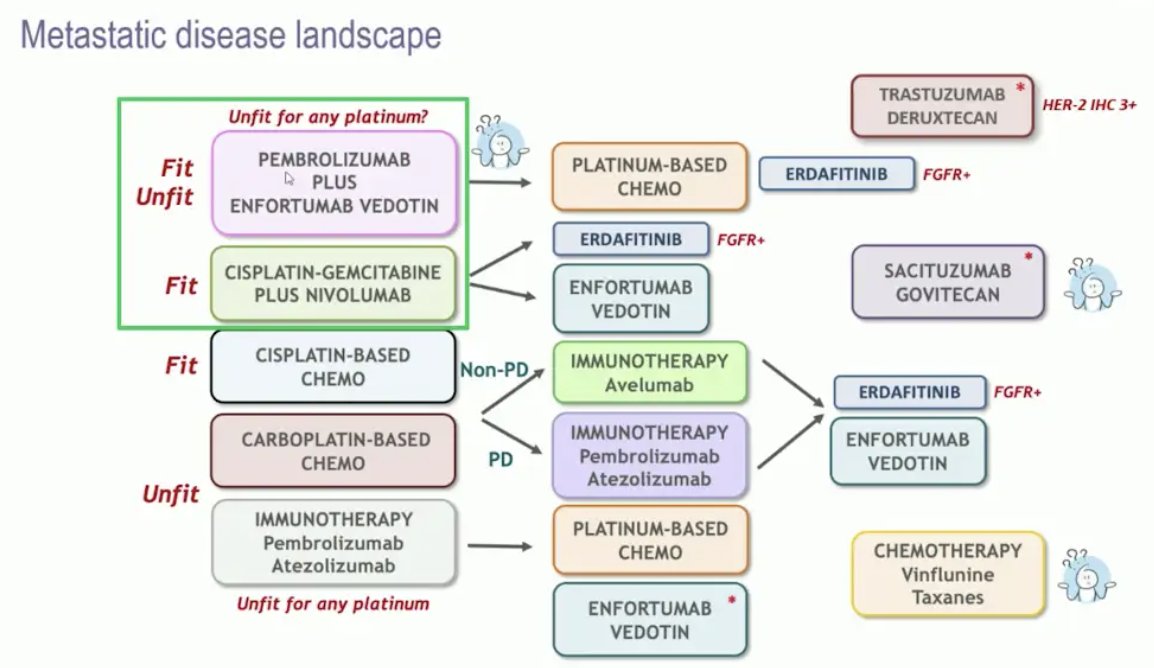
Particularly in the first line setting, this has changed drastically since ESMO 2023 when both CheckMate 9012 (gemcitabine + cisplatin + nivolumab) and EV-3023 (enfortumab vedotin + pembrolizumab) were presented. There are now many novel agents and combinations, as well as antibody-drug conjugates:

One antibody-drug conjugate of particular interest is disitamab vedotin targeting HER-2. Previous phase 2 trials have shown a response rate of ~50% with disitamab vedotin. Several ongoing trials are assessing this agent, specifically RC48G001 (cohort C data being presented at ESMO 2024) and SGNDV-001:
Another HER-2 antibody-drug conjugate, trastuzumab deruxtecan, was evaluated in the pan-tumor trial DESTINY (n = 267), with bladder cancer patients in this trial (n = 41) having an objective response rate of 39.0% and one patient having a complete response. Secondary to this data, on April 5, 2024, the FDA granted trastuzumab deruxtecan accelerated approval for unresectable or metastatic HER-2 positive solid tumors.
With regards to the Trop-2 antibody-drug conjugates, sacituzumab govitecan is being assessed in the phase 3 TROPiCS-04 trial following first-line platinum-based chemotherapy in the advanced or metastatic urothelial carcinoma setting versus investigator’s choice of chemotherapy. However, a press release from May 30, 2024, stated that the trial did not meet its primary endpoint of overall survival in the intention to treat population. Datopotamab deruxtecan is another anti-Trop-2 antibody-drug conjugate that was tested in the pan-tumor TROPION trial (n = 32), with a noted objective response rate of 19.2%. The following table highlights antibody-drug conjugate combinations with IO in urothelial carcinoma: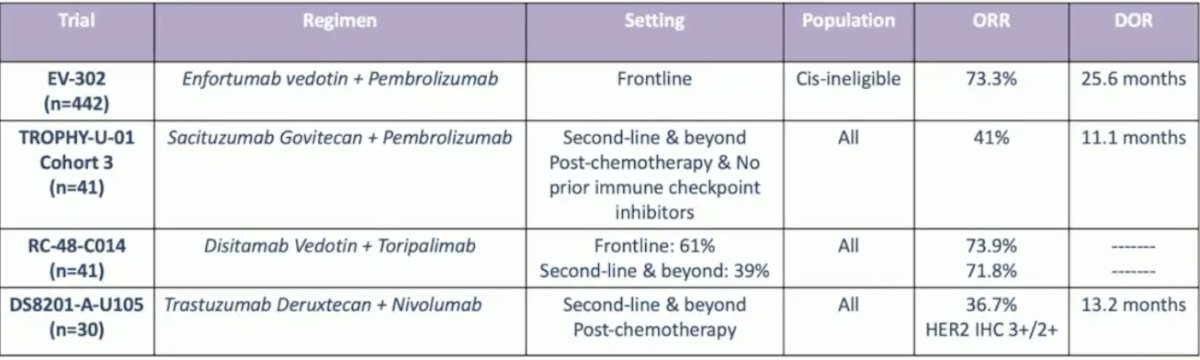
Dr. Perez-Valderrama concluded her presentation discussing how to build on standard therapies by assessing novel agents and promising combinations with the following take-home points:
- There are many promising agents being evaluated in early trials in renal and urothelial carcinoma. However, despite these promising results, how many will become practice-changing trials? We need to be cautious with treatment until we have data
- There are several questions/statements to consider:
- Can we extrapolate data from patients included in clinical trials to patients in the real-world setting? Are they the same population?
- We need mature overall survival data to confirm the real benefit of a novel agent or combination regimen
- We need to know the long-term toxicities and how they will impact the quality of life for patients.
- We need to incorporate local therapies in those patients with complete and durable responses. How will this impact overall survival and quality of life?
- Early Access of new alternatives is difficult in some countries
- How will new data in the peri-operative setting impact the tumor biology in metastatic disease?
Presented by: Begona Perez-Valderrama, MD, Hospital Universitario Virgen del Rocio, Seville, Spain
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
References:
- Choueiri TK, McDermott DF, Merchan J, et al. Belzutifan plus cabozantinib for patients with advanced clear cell renal cell carcinoma previously treated with immunotherapy: An open-label, single-arm, phase 2 study. Lancet Oncol. 2023 May;24(5):553-562.
- Van der Heijden MS, Sonpavde G, Powles T, et al. Nivolumab plus Gemcitabine-Cisplatin in Advanced Urothelial Carcinoma. N Engl J Med. 2023 Nov 9;389(19):1778-1789.
- Powles T, Valderrama BP, Gupta S, et al. Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. N Engl J Med. 2024 Mar 7;390(10)875-888.


