(UroToday.com) The 2024 European Society of Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 17th was host to the session Mini oral session: GU tumours, non-prostate. Dr. Aristotelis Bamias was the invited discussant to summarize and critically appraised the presentations by Drs Vadim S. Koshkin, Thomas B. Powles, and Matthew D. Galsky exploring different treatment options for locally advanced and metastatic urothelial (la/mUC) carcinoma.
Dr. Bamias began by discussing the presentation by Dr. Koshkin, who presented the final analysis of efficacy and safety from the phase 2 study of futibatinib plus pembrolizumab in patients with locally advanced/metastatic urothelial carcinoma (la/mUC). This trial aligns with the most recent progress in the field, exploring new treatment options in the first-line setting for la/mUC. Specifically, it investigates the use of an FGFR inhibitor in combination with an immune checkpoint inhibitor (anti-PD-1) in patients with FGFR alterations. The aim is likely to improve outcomes compared to the new standard of care for la/mUC, which is Enfortumab Vedotin and Pembrolizumab (EV+P). This combination has shown an unprecedented advantage in overall survival compared to standard-of-care chemotherapy.1
Dr. Bamias congratulated the investigators for evaluating biomarker targeting in all three trials. The investigators assessed three different targets: fibroblast growth factor receptors 1–4 (FGFR1‒4), Nectin-4, and HER-2. A summary of the selection criteria for each trial and the percentage of patients harboring these biomarkers is shown in the table below.
Notably, Nectin-4 was present in almost all patients (100%) (EV-302), FGFR3 mutations or FGFR1-4 rearrangements were present in 20% of the patients (Koshkin’s), and HER-2 was present in 20% of the population in this study (Galsky’s).
Beginning with FGFR, this has already been very well established as an approved biomarker, with Level I evidence supporting its use in patient selection for:
- Erdafitinib: FGFR3 mutations or fusions (2)
- Futibatinib: FGFR3 mutations or FGFR1-4 rearrangements/fusions
However, two questions remain unanswered with this biomarker:
- Does the inclusion of FGFR1/4 aberrations make any difference compared to FGFR3 mutations or fusions?
- Can FGFR aberrations be used not only as targeting biomarkers but also as predictive biomarkers?
Interestingly, an analysis of UC (n=126) showed that approximately 67% of patients had no FGFR mutations, fusions, or rearrangements. Activating FGFR3 mutations were present in 15% of patients, while FGFR1 amplifications were found in only 7% as illustrated below:
This trial, evaluating futibatinib plus pembrolizumab, demonstrated an ORR of 47.1% in Cohort A (patients with FGFR3 mutations or FGFR1-4 rearrangements/fusions) compared to 26.9% in Cohort B (patients with wild-type or other FGFR or non-FGFR aberrant tumors). Similarly, the median PFS was more than double in Cohort A, supporting the use of FGFR3 mutations or FGFR1-4 rearrangements/fusions as biomarkers to guide treatment in the first-line setting for la/mUC patients.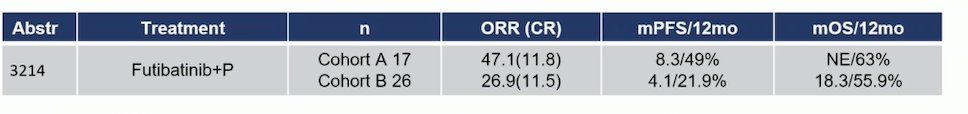
Dr. Bamias then discussed the presentation by Dr. Powles. In this exploratory biomarker analysis of the EV-302 study, the researchers investigated the relationship between Nectin-4 expression and oncological outcomes. They conducted a retrospective assessment of Nectin-4 expression.
Nectin-4 is expressed in almost all or all UC, with three levels of expression proposed. In this exploratory biomarker analysis of the EV-302 trial, 75% of patients were in the high-expression group. The investigators found a consistent PFS benefit with EV + Pembrolizumab in both Nectin-4 H-score subgroups: <275 and ≥275. The overall response rate was 71.8% in the EV+P arm with Nectin-4 high expression and 63.6% in the Nectin-4 low expression group (<275).
Lastly, in bladder cancer, HER2 overexpression has generally been associated with tumor progression and poor prognosis, suggesting it could be a predictive biomarker in la/mUC. Similarly, in breast and gastric cancers, there is growing evidence of HER2 overexpression correlating with poor outcomes and more aggressive disease.
In the study presented by Dr. Galsky, Disitamab Vedotin (DV; RC48-ADC), an investigational antibody-drug conjugate comprising a fully humanized HER2-directed monoclonal antibody, was evaluated in patients with HER2-high or HER2-low expressing tumors. The confirmed overall response rate (ORR) was 75% for the overall cohort. Patients in both the HER2-positive and HER2-low groups responded to DV+P treatment. In the HER2-positive group, the ORR was 66.7%, while in the HER2-low group, it was 78.6%. This raises the question: is there a real benefit to using HER2 as a targeting biomarker?
Is it possible to improve over the standard of care Enfortumab vedotin + Pembrolizumab using these targeted biomarkers?
With FGFR, there is a rationale for combining anti-FGFR agents with anti-PD1/L1 agents in mUC with FGFR aberrations, as this approach has shown to enhance antitumor immunity in preclinical models (4). Erdafitinib remains active post-treatment with an ICI (2). However, there is no evidence to date that Pembrolizumab is effective in FGFR3 aberrant tumors. How does it compare to EV+P and Gemcitabine + Cisplatin + Nivolumab?
In Cohort A of the Futibatinib trial (patients with FGFR3 mutations or FGFR1-4 rearrangements/fusions), the ORR was 47%, and the median PFS was 8.3 months. In the EV-302 trial, the ORR was 66.7%, and the median PFS was 12.5 months. In the CheckMate 901 trial, the ORR was 57.6%, and the median PFS was 7.9 months. (5) It compares fairly with both trials. However, the limitation of the small cohort sample (n=17) has to be taken into consideration.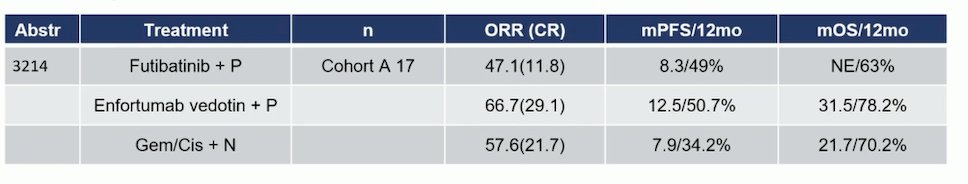
Furthermore, the EV-302 biomarker exploratory analysis provides the most scientifically robust insight into the role of Nectin-4 expression for patient selection in the first-line treatment of mUC. The negative results of this study apply specifically to treatment with EV+P and confirm that there is no need to measure Nectin-4 or PD-L1 expression to guide treatment with EV+P.
Comparing Disitamab monotherapy with EV, data from patients with mUC pre-treated with chemotherapy and those with IHC+3/IHC+2 and FISH-positive status showed an ORR of 62.2% for Disitamab and 40.6% for EV. This suggests that Disitamab is a promising therapeutic target in la/mUC. However, Dr. Galsky’s study indicated that DV+P showed encouraging preliminary activity in both HER2-positive and HER2-low patients, but the role of HER2 as a targeting biomarker remains uncertain.
Dr. Bamias concluded his critical appraisal of these three presentations by stating:
- FGFR inhibition represents the most advanced targeted strategy in la/mUC, but its role in the first-line setting requires further study.
- Selection by Nectin-4 or PD-L1 is not necessary for treatment with EV+P. Subgroup analyses of EV-302 by FGFR aberration and HER2 expression may be valuable, and he encouraged the investigators to pursue these analyses.
- The role of HER2 as a target for the treatment of mUC needs to be clarified. Disitamab warrants further research in mUC but not just in the first-line setting.
- Recent progress and ongoing research in the systemic therapy of mUC are unprecedented.
Presented by: Aristotelis Bamias, MD, National and Kapodistrian University of Athens, Athens, Greece.
Written by: Julian Chavarriaga, MD – Urologic Oncologist at Cancer Treatment and Research Center (CTIC) Luis Carlos Sarmiento Angulo Foundation via Society of Urologic Oncology (SUO) Fellow at The University of Toronto. @chavarriagaj on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 17th
References:- Powles T, Valderrama BP, Gupta S, Bedke J, Kikuchi E, Hoffman-Censits J, Iyer G, Vulsteke C, Park SH, Shin SJ, Castellano D, Fornarini G, Li JR, Gümüş M, Mar N, Loriot Y, Fléchon A, Duran I, Drakaki A, Narayanan S, Yu X, Gorla S, Homet Moreno B, van der Heijden MS; EV-302 Trial Investigators. Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. N Engl J Med. 2024 Mar 7;390(10):875-888. doi: 10.1056/NEJMoa2312117. PMID: 38446675.
- Loriot Y, Matsubara N, Park SH, Huddart RA, Burgess EF, Houede N, Banek S, Guadalupi V, Ku JH, Valderrama BP, Tran B, Triantos S, Kean Y, Akapame S, Deprince K, Mukhopadhyay S, Stone NL, Siefker-Radtke AO; THOR Cohort 1 Investigators. Erdafitinib or Chemotherapy in Advanced or Metastatic Urothelial Carcinoma. N Engl J Med. 2023 Nov 23;389(21):1961-1971. doi: 10.1056/NEJMoa2308849. Epub 2023 Oct 21. PMID: 37870920.
- Helsten T, Elkin S, Arthur E, Tomson BN, Carter J, Kurzrock R. The FGFR Landscape in Cancer: Analysis of 4,853 Tumors by Next-Generation Sequencing. Clin Cancer Res. 2016 Jan 1;22(1):259-67. doi: 10.1158/1078-0432.CCR-14-3212. Epub 2015 Sep 15. PMID: 26373574.
- Palakurthi S, Kuraguchi M, Zacharek SJ, Zudaire E, Huang W, Bonal DM, Liu J, Dhaneshwar A, DePeaux K, Gowaski MR, Bailey D, Regan SN, Ivanova E, Ferrante C, English JM, Khosla A, Beck AH, Rytlewski JA, Sanders C, Laquerre S, Bittinger MA, Kirschmeier PT, Packman K, Janne PA, Moy C, Wong KK, Verona RI, Lorenzi MV. The Combined Effect of FGFR Inhibition and PD-1 Blockade Promotes Tumor-Intrinsic Induction of Antitumor Immunity. Cancer Immunol Res. 2019 Sep;7(9):1457-1471. doi: 10.1158/2326-6066.CIR-18-0595. Epub 2019 Jul 22. PMID: 31331945.
- van der Heijden MS, Sonpavde G, Powles T, Necchi A, Burotto M, Schenker M, Sade JP, Bamias A, Beuzeboc P, Bedke J, Oldenburg J, Chatta G, Ürün Y, Ye D, He Z, Valderrama BP, Ku JH, Tomita Y, Filian J, Wang L, Purcea D, Patel MY, Nasroulah F, Galsky MD; CheckMate 901 Trial Investigators. Nivolumab plus Gemcitabine-Cisplatin in Advanced Urothelial Carcinoma. N Engl J Med. 2023 Nov 9;389(19):1778-1789. doi: 10.1056/NEJMoa2309863. Epub 2023 Oct 22. PMID: 37870949.


