(UroToday.com) The 2024 European Society for Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 16th, 2024 was host to a genitourinary cancers poster session. Dr. Aleksander Antoniewicz presented the results of an interim analysis of a phase I study evaluating trifunctional anti-EpCAM/CD3 bsAb catumaxomab intravesically for intermediate- and high-risk non-muscle invasive bladder cancer (NMIBC).
CATUNIBLA is a phase I trial (NCT04819399) that was designed to determine the safety, pharmacokinetics/pharmacodynamics, the recommended phase II dose, and first efficacy signs of catumaxomab (CAT) in intermediate- and high-risk NMIBC.
The study design is summarized below:
Patients with newly diagnosed or relapsed/refractory intermediate-, high-, or highest-risk NMIBC, per the EAU guidelines, after the 1st TURBT were eligible. Patients received 6 x once weekly intravesical (2-hour) CAT instillations at doses of 50, 70, or 100 μg in 50 mL via a urinary catheter. The time between TURBT and the first CAT instillation was required to be between 1 and 3 weeks. After completion of CAT therapy, a 2nd TURBT and adjuvant instillations were performed according to standard of care. Human anti-mouse antibody (HAMA) responses, pharmacokinetics, cytokines, and urinary leukocytes were quantified. EpCAM+ tumor cells were determined in urine and tumor tissue. The median follow-up of all treated patients was 13.7 months (range: 5–35.2).
In part 1 (dose escalation), 10 patients with high-risk NMIBC were treated with 50 μg (cohort 1), 70 μg (cohort 2), or 100 μg (cohort 3) per instillation. No dose-limiting toxicity (DLT) occurred. The maximum tolerated dose (MTD) was not reached. At the lowest dose, 2/3 patients experienced a recurrence on day 180 indicating insufficient drug dose. In contrast, all 3 patients in cohort 2 receiving the complete treatment cycle remained in complete response until day 720. Thus, the recommended part II dose was set to 70 μg. Likewise, no recurrences occurred at the highest dose of 100 μg. Tissue samples of all 10 patients (100%) were positive for EpCAM.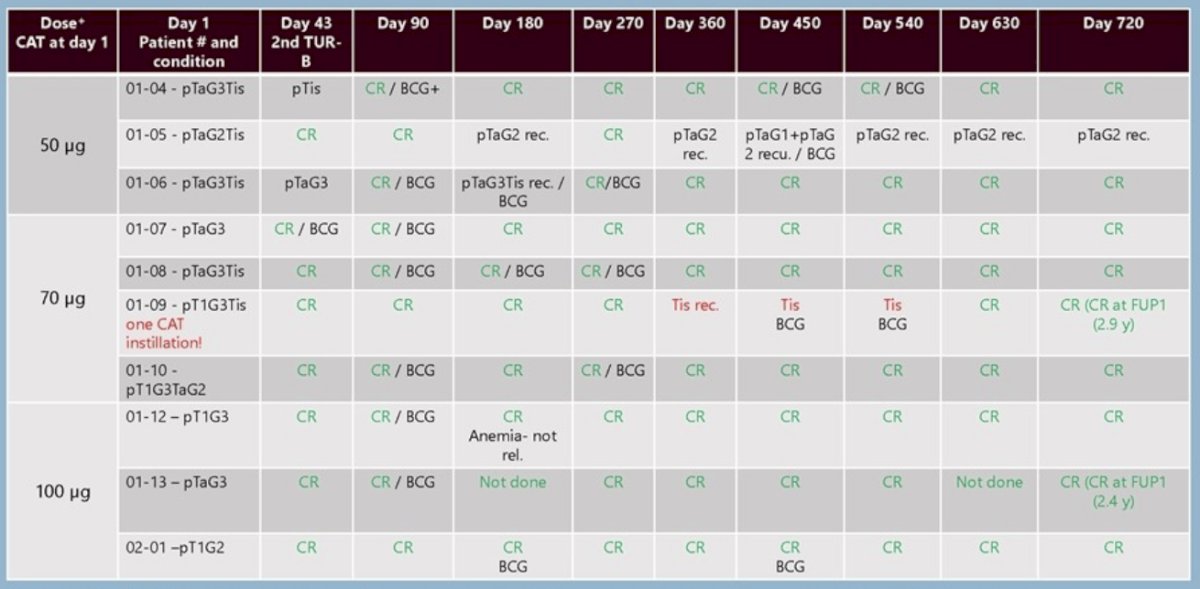
In part II of the study, a further 17 patients (2 dropouts due to protocol violations were excluded) with either high/highest-risk (71%) or intermediate-risk (29%) NMIBC were treated with the recommended dose of 70 μg. Patients did not experience any drug-related serious adverse events, but procedure-related urinary tract infection was common. All CAT-induced adverse events were mild or moderate (grade 1-2) and systemic CAT was not quantifiable.
Accordingly, serum cytokines were typically not detectable. 16/21 (76%) patients were in complete response at 2nd TURBT (dav 43) and 7/10 (70%) patients with Tis achieved a complete response before BCG therapy. During the 13.7 months median follow-up, 2/20 (10%) patients had tumor recurrences. There was one treatment failure (resulting in cystectomy), but no tumor progression. The overall response rate of the combination of TURBTs, CAT, and BCG was 96%.
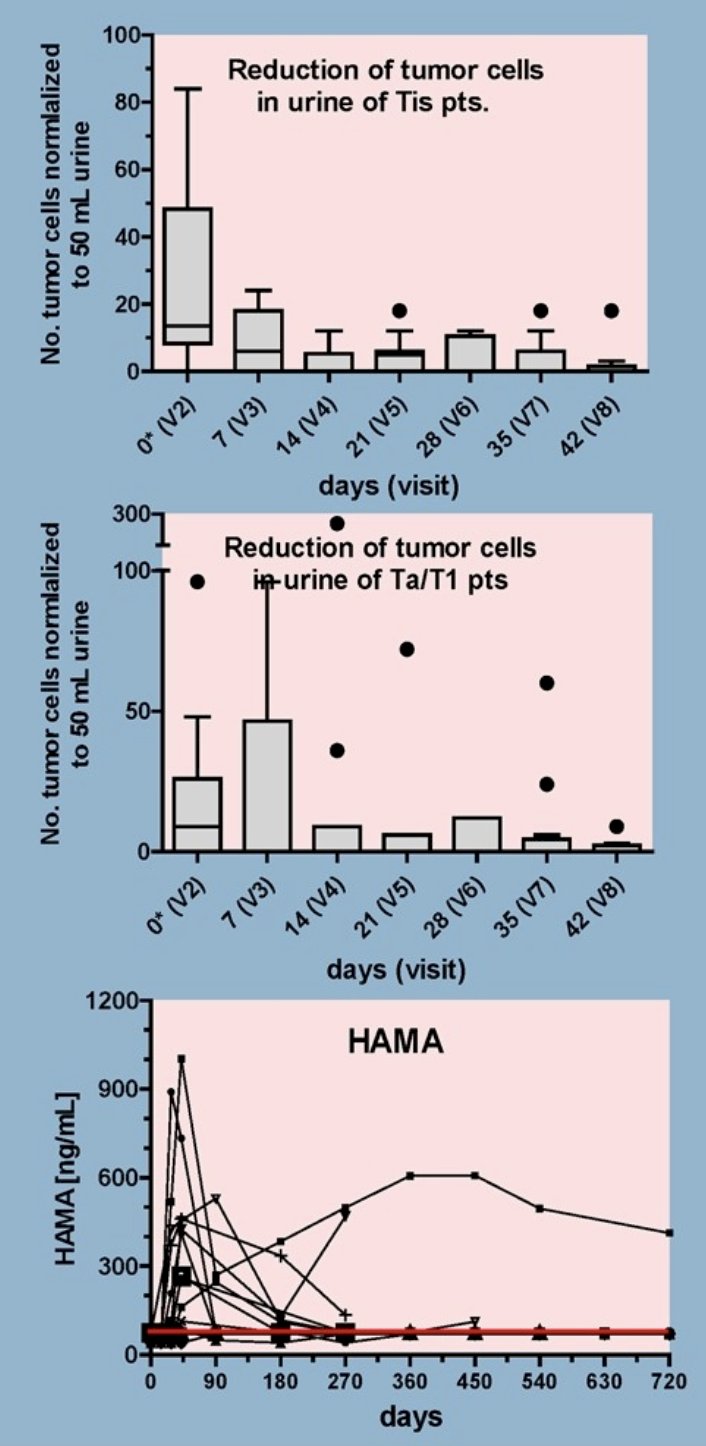
Reduction of tumor cells in urine and HAMA response:
- The number of tumor cells in the urine was analysed by ICH staining (EpCAM, cytokeratin) before and during CAT therapy at the indicated visits. Tumor cells decreased to zero in median at visit 8 (day 42) in both populations with Tis (upper figure) and with Ta/T1 tumors (middle figure). Results are depicted as whiskers turkey box plots. Human anti-mouse antibody (HAMA) responses were developed in 11/27 (41%) patients receiving ≥1 CAT instillation (lower figure). HAMA was generally low (≤1000 ng/mL) and transient peaking around day 42.
Increase of leukocytes in urine:
- The number of leukocytes in the urine was determined by IHC staining (CD45) before (Va), 6 hours (Vb), or 24 hours (Vc) after CAT instillations for visits V2-V5. At V6-V8 only pre-CAT samples were analysed. Typically, leukocytes peaked after each CAT application but decreased to normal before the next instillation. This was observed for Tis (single or in combination) patients (left figure) as well as for Ta/T1 patients (right figure). Arrows indicate CAT instillations

Dr. Antoniewicz concluded as follows:
- Intravesical administration of the trifunctional, bi-specific antibody, Catumaxomab, is well-tolerated in patients with NMIBC
- Catumaxomab does not enter systemic circulation and elicits only low and transient immunogenicity
- The maximum tolerated dose was not reached, and the recommended phase Il dose was determined at 70 μg
- Catumaxomab instillations induce a transient increase of local leukocytes and a strong reduction of EpCAM-positive tumor cells in urine
- 76% of patients were in complete response at 2nd TURBT, and 70% of Tis patients achieved a complete response before BCG therapy (70 μg dose)
- 10% of patients had a tumor recurrence during a median follow-up of 13.7 months (70 μg dose)
- The safety data and efficacy signals are promising
Written by: Rashid Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.


